Need to write up a student experiment report for QCAA Chemistry but have no idea where to start?
If you’re unsure about how you’re meant to approach this Chemistry IA, don’t worry! We’ll take you through writing up the student experiment report for Chemistry section by section, so you know exactly what needs to go where.
Ready to get started? Let’s go!
What is a Student Experiment?
How do you write up a Student Experiment Report for Chemistry?
Chemistry Student Experiment Report Structure
What is a Student Experiment?
A Student Experiment is essentially a research report based on an experiment that is conducted by you (the student).
The purpose of a Student Experiment is to refine, extend and/or redirect an existing experiment in order to answer a new, but still related, research question.
Once the experiment has been conducted and the required data has been gathered, the data is then processed, analysed and used to draw conclusions to answer your research question.
How do you write up a Student Experiment Report for QCAA Chemistry?
Ultimately, the findings of your experiment and all the research and analysis you’ve conducted is presented in the form of a scientific report that is 1500 to 2000 words in length.
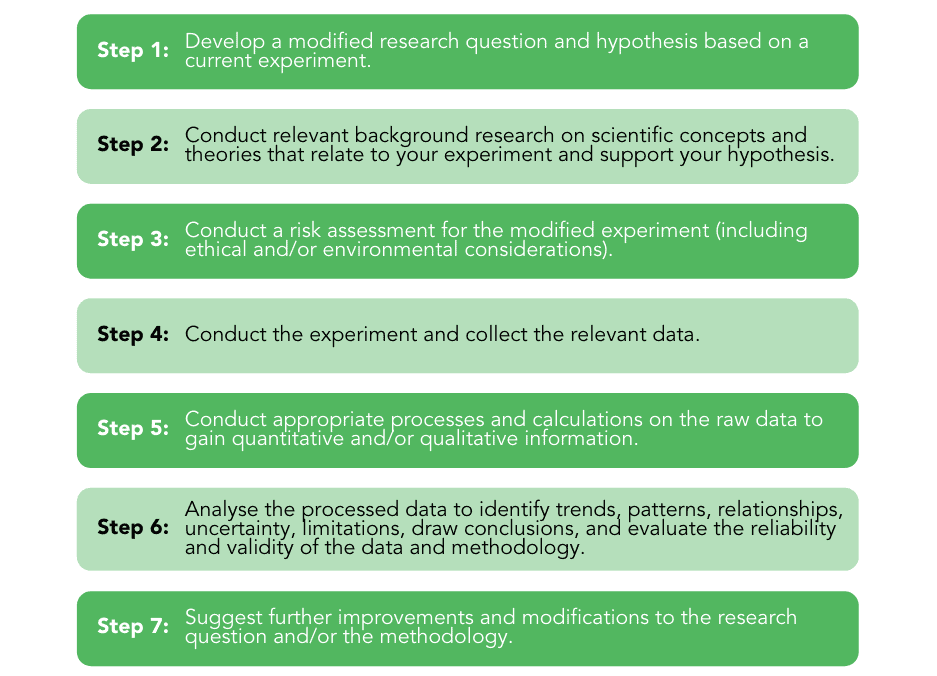
In order to create a comprehensive scientific report, there are 7 steps to follow. These include:
Although development of the research question, methodology, risk assessment and the actual experiment can be done collaboratively, all other aspects need to be completed individually. And for this, we have a guide of what exactly each section of the QCAA Chemistry Student Experiment needs to meet!
Chemistry Student Experiment Report Structure
#1: Title
Your title should be a comprehensive but short description about your report that lets the reader know exactly what it’s about. It might get a little lengthy, but it’s important that your title is specific and clear.
For example: Effect of Hydrochloric Acid concentration on the volume of standard Sodium Carbonate solution required to reach the first equivalence point when titrated
#2: Rationale
This is where the majority of the information regarding the scientific concepts, theories and processes related to your experiment should be found. Much of this information will not be provided with the original experiment, so it’s up to you to find, utilise and reference relevant and insightful information.
Figures and formulas can be useful in this section to help explain the (possibly) complex theories you may be explaining.
After providing this context information, apply it in order to justify and support your hypothesis, which will also be included as part of your rationale. Remember to reference scientific literature here!
For example: According to the Bronsted-Lowry Theory, acids are species that can donate protons (H+) and bases are substances that accept them. pH can be described using hydrogen ion concentration, as they are linked logarithmically. For example, a solution of pH 4 is ten times more acidic than a solution of pH 5. Substances that can donate more than one proton per molecule are known as polyprotic acids. Strong acids and bases dissociate completely in water, whereas weak acids and bases only dissociate partially… This means that there will be two equivalence points. The first equivalence point occurs at around pH = 3.7. The second equivalence point occurs at around pH = 8.3.
#3: Original Experiment
This should just be a brief summary of what the original experiment consisted of, especially in terms of its aim. You should also reference where the methodology for the original experiment is from here.
For example: The original experiment investigated the volume of standard sodium carbonate solution (of a known concentration) needed to reach the first equivalence point when titrated with hydrochloric acid (of an unknown concentration).
#4: Research Question
Here, you state your research question. However, remember to be specific — even more specific than you were in your title.
State known volumes, concentrations or other factors if applicable to your question. Make sure the independent variable and independent variable are both clearly stated!
#5: Modifications to Methodology
In this section of the QCAA Chemistry Student experiment, you will need to state the modifications you’ve made to the methodology and explain how they will extend, refine, or redirect the experiment.
Extend
To extend an experiment means you will be altering the methodology in order to overcome certain limitations of the scope, or how the data can be applied. This typically allows for further investigations and to understand what is happening in the experiment better.
Refine
Refining your experiment essentially means you are changing the methodology in order to gather more accurate or precise data, especially if the data is not specific enough.
Redirect
This means that you’ll be adapting the methodology to develop more insight into what was observed initially in the original experiment.
#6: Management of Risks
Include here any safety considerations you had to make when designing the experiment, whether that be in terms of safety gear, correctly disposing of toxic substances or adherence to standard laboratory safety procedures.
Also include any steps you will take to manage the possible risks your experiment poses!
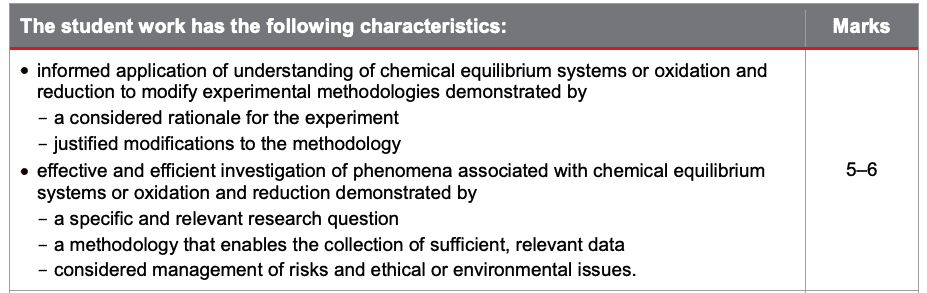
Up until this point in your report, most of the presented information demonstrates your research and planning skills. Let’s have a look at what you need to score top marks in this section of the criteria:
Make note of the fact that you need a considered rationale, justified modifications, a specific and relevant research question, considered management of risks, etc. in order to score high marks.
#7: Raw Data
Present your raw data with the associated uncertainties here. You may also want to include any qualitative observations.
You may present your data in the form of a table listing the various trials conducted.
#8: Processing of Data
Firstly, you should state the ways in which you processed the data in order to analyse it effectively (calculating the average, calculating percentage uncertainty etc.).
Then, include all the relevant formulas and calculations for one of your data sets (i.e., just for the titration with 0.1M HCl). As you’ll probably have a page limit, you won’t be able to include all your calculations within the main body of your report — the rest will go in the appendix and be referred to later on if required.
Also include in this section your calculations of the uncertainty of the instruments and equipment you used as well as any relevant graphs (specifically the ones you plan on referring to in the analysis section).
Remember that QCAA requires all tables and graphs to have titles. It’s a good idea to also double-check all your calculations, units and symbols.
#9: Trends, Patterns and Relationships
Here is where you analyse your processed data to identify trends, patterns and relationships in order to reach a justified conclusion. You can do this by going through the most insightful graphs, tables and/or calculations and explaining what they suggest and why this is significant.
Make sure to be specific in terms of the values! Provide reasoning for any trends and/or inconsistencies if possible.
For example: Table 3 demonstrates that the number of moles of hydrochloric acid and sodium hydroxide were not equal at the equivalence point in the 0.2M titration (18.53% error), suggesting there was some error in the titration process.
#10: Limitations of Evidence
In this section, provide sources of error, unreliability and uncertainty in your experimental methodology — describe how they impacted your results, and suggest appropriate improvements or extensions for your student experiment.
Be specific about whether they are random or systematic errors.
For example:
Sources of Error Suggested Improvements of Extensions Equipment used to measure the standard sodium carbonate solution is imprecise (systematic error) (0.02g/L). Equipment with higher levels of precision could be used.
#11: Conclusion
Your conclusion should link directly to your research question! It should summarise your major findings, comment on the accuracy of the experimental process and results and provide recommendations for improvement or extension.
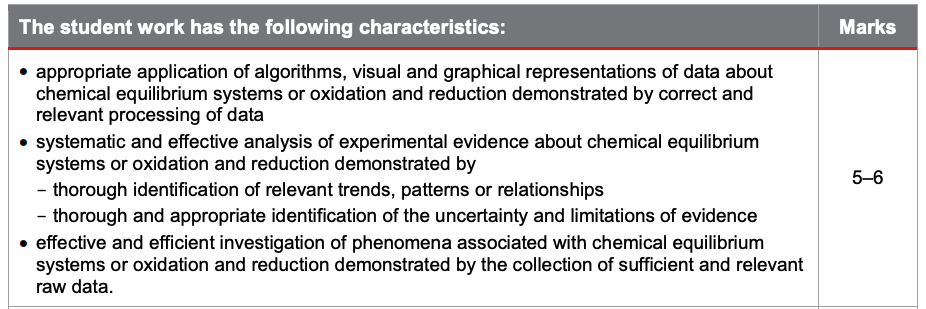
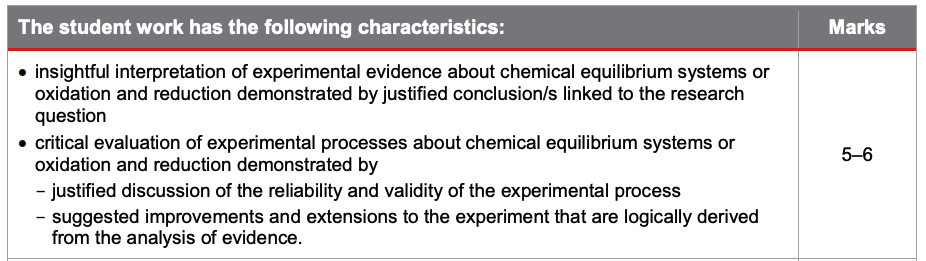
The second half of your report consists of analysis of evidence as well as interpretation and evaluation. Let’s check out the criteria relating to this:
Again, in order to achieve those top marks, ensure that you’ve included appropriate application of algorithms, thorough identification of trends, insightful interpretation of experimental evidence, logically derived improvements and extensions etc.
#12: Reference List
Remember to cite any scientific literature you may have used in your report. Make sure it is in the style your school/teacher has requested.
#13: Appendix
Include any graphs/tables that were not included in the main body of the report here. As mentioned previously, you your tables may include any other calculations you’ve made that have been excluded from the main body of the report.
There you have it!
You’ve now got everything you need to know about what you need to cover in your Chemistry student experiment report. The best thing to remember is to always refer back to your marking guide and use it as a checklist, to ensure you’ve included everything required of you!
On the hunt for other QCAA Chemistry resources?
Want to see how you really went in the IA1 data test? Check out our QCE Cohort Comparison Tool to check where you sit compared to your peers!!
We’ve got plenty more practice questions for you to use to revise previous content from throughout the year! Check them out:
- Unit 3 Chemistry Data Test IA1 Practice Questions
- Practice Questions for Unit 3 & 4 Chemistry External Assessment
- Multiple Choice Practice Questions for Unit 3 & 4 Chemistry External Assessment
- Download QCAA Chemistry Practice Exam for External Assessment Revision
You’ll also want to have a look at our nifty guides for working on your QCAA Chemistry assessments below:
- The Ultimate Guide to Conducting a QCAA Chemistry Research Investigation
- How to Ace Your External Assessment for QCAA Chemistry
- The Ultimate Guide to QCAA Chemistry Unit 3: Equilibrium, Acids and Redox Reactions
- The Ultimate Guide to QCAA Chemistry Unit 4: Structure, Synthesis and Design
Also studying Physics? We recommend checking out our Ultimate Guide to Unit 4 of QCAA Physics: Revolutions in Modern Physics!
Are you looking for some extra guidance with the Student Experiment for QCAA Chemistry?
We have an incredible team of QLD tutors and mentors!
We can help you master the Chemistry syllabus and ace your upcoming Chemistry assessments with personalised lessons conducted one-on-one in your home or online!
We’ve supported over 8,000 students over the last 11 years, and on average our students score mark improvements of over 20%!
Looking for tutoring and live in a regional area? We have online tutoring options available or face-to-face tutoring for the Townsville area!
To find out more and get started with an inspirational QCE tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Yalindi Binduhewa is an Art of Smart tutor based in Queensland and was part of the very first cohort to go through the ATAR system, so she knows exactly how fun and enjoyable it can be. She is currently studying a Bachelor of Medical Imaging (Honours) at QUT and is loving it. When she’s not doing uni-related stuff or tutoring, she’s hanging out with her friends, rewatching a show for the 100th time, or trying out new crafty projects and discovering that she doesn’t have a talent for everything.