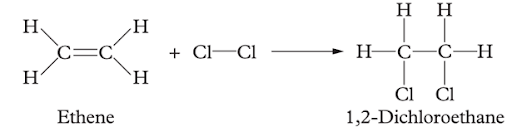It can be hard to find good practice questions to help you prepare for your QCAA Chemistry External Exam (EA). To study well, you’ll obviously want practice questions with answers to refer back to, so you know where to improve!
Lucky for you, we’ve got exactly that. Once you’ve had a go at all 25 Chemistry EA practice questions, you’ll be able to download the solutions.
Keen to begin studying? Let’s go!
Chemistry EA Practice Questions
Question 1 (1 mark)
Explain what it means for a state of dynamic equilibrium to have been reached in a reversible reaction.
Question 2 (3 marks)
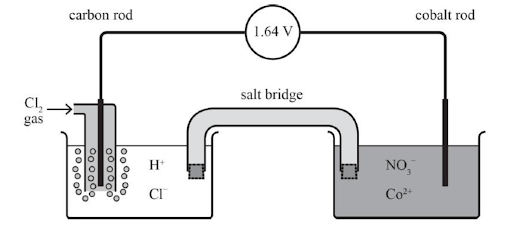
The diagram below is an electrochemical cell. Label the following on the diagram:
(a) The positive electrode (cathode) and the negative electrode (anode)
(b) The direction of the flow of electrons
(c) The direction of the movement of the ions in the salt bridge
Question 3 (3 marks)
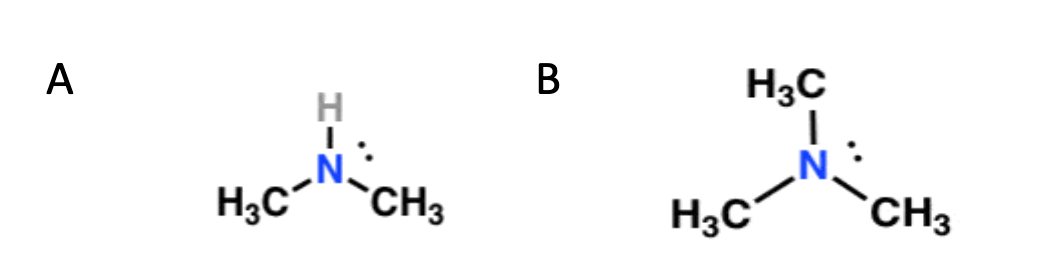
Shown below are two amines. Determine which structure contains a secondary carbon and which structure contains a tertiary carbon. Justify your answer.
Image sourced from Master Organic Chemistry
Question 4 (2 marks)
Calculate the concentration of hydroxide ions (OH–) at pH 10.5. Show your working.
Question 5 (2 marks)
Calculate the concentration of hydrogen ions (H+) at pH 2.7. Show your working.
Question 6 (3 marks)
If 10.25 mL of a 0.8 M standardised solution of sodium hydroxide (NaOH) is required to neutralise 9.00 mL of sulphuric acid (H2SO4), calculate the concentration of the sulphuric acid solution (in mol/L). Show your working.
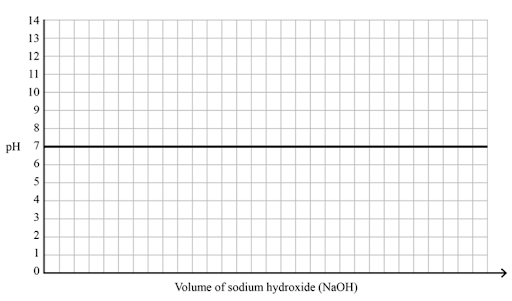
Question 7 (4 marks)
On the axes below, sketch the titration curve when 0.2 M hydrochloric acid (HCL) is titrated with 0.2 M sodium hydroxide (NaOH) and label the:
(a) initial pH of the acid
(b) equivalence point
(c) half-equivalence point
(d) buffer region
Image sourced from QCAA Chemistry Public Use Practice Paper
Question 8 (2 marks)
Calculate the percentage yield (by mass) for hydrogen in the following reaction:
Mg(s) + H2SO4(aq) → H2(g) + MgSO4(aq)
Question 9 (2 marks)
If Kc is larger than 10-3 is there significantly more reactant or product species present at the equilibrium? Explain your answer with reference to the Kc formula.
Question 10 (3 marks)
Explain how soap is able to remove grease. Refer to intermolecular forces in your answer.
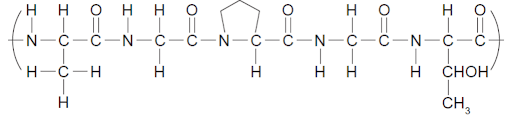
Question 11 (2 marks)
Determine the name of the polypeptide chain below (using the amino acid abbreviations).
Question 12 (2 marks)
Explain the relationship between the strength of an acid and electrical conductivity.
Question 13 (1 mark)
What types of electrochemical cells use energy from an external source?
Question 14 (4 marks)
Distinguish between galvanic and electrochemical cells.
Question 15 (2 marks)
What is the definition of a strong acid in terms of its Ka? Give an example of a strong acid.
Question 16 (2 marks)
What is the definition of a strong base in terms of its Kb? Give an example of a strong base.
Question 17 (3 marks)
Distinguish between strong acids/bases and weak acids/bases.
Question 18 (2 marks)
Define the cell potential and provide the formula associated with it.
Question 19 (2 marks)
Explain the relationship between pH and hydrogen ion concentration [H+].
Question 20 (4 marks)
Sodium carbonate (Na2CO3) is a relatively strong base with a pH of approximately 11-12. Hydrochloric acid (HCl) is a strong acid with a pH of approximately 1-2. The neutralisation reaction of hydrochloric acid and sodium carbonate occurs according to equation (1):
Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq)+ H2O(l) + CO2(g)
However, as the carbonate ion in sodium carbonate can accept two H+ ions in an acid-base reaction, the reaction occurs in two successive steps. The first step is given below.
Na2CO3(aq) + HCl(aq)→ NaHCO3(aq) + NaCl(aq)
(a) Determine what the second step will be.
(b) Deduce what this means in terms of the number of equivalence points.
Question 21 (3 marks)
Differentiate between random and systematic error and give an example for each that could occur during a titration reaction.
Question 22 (2 marks)
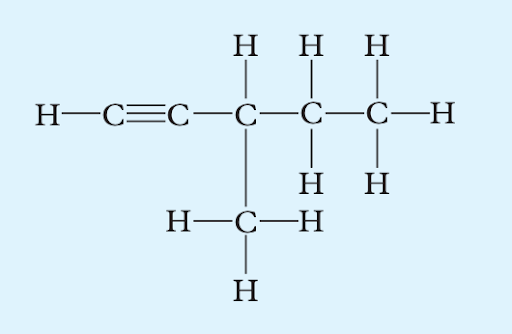
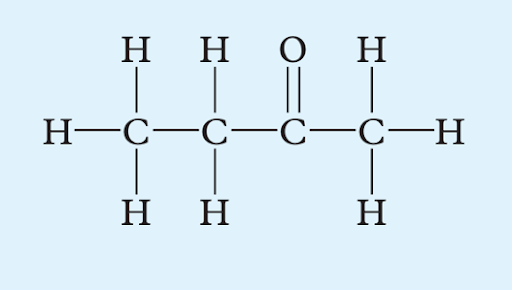
Apply IUPAC rules to name the following structures:
(a)
Image sourced from Oxford Chemistry Units 3 & 4
(b)
Image sourced from Oxford Chemistry Units 3 & 4
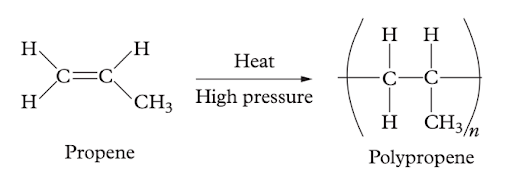
Question 23 (4 marks)
For each of the following reactions, determine what kind of organic reaction has occurred (be specific).
(a)
Image sourced from Oxford Chemistry Units 3 & 4
(b)
Image sourced from Oxford Chemistry Units 3 & 4
(c)
Image sourced from Oxford Chemistry Units 3 & 4
(d)
Image sourced from Oxford Chemistry Units 3 & 4
Question 24 (4 marks)
Explain how glucose monomers combine to form a branched glycogen polymer.
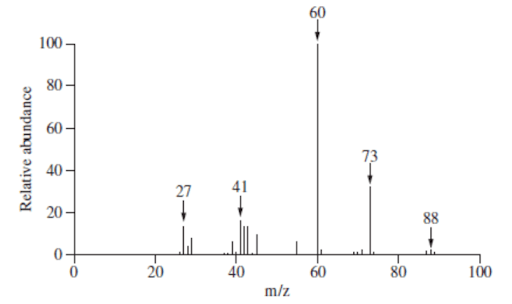
Question 25 (4 marks)
Identify the organic compound from the mass spectrum below.
Solutions to Chemistry EA Practice Questions
Click here to download the solutions to the 25 practice questions above!
On the hunt for other QCAA Chemistry resources?
Stressing about the externals and your ATAR? Check where you sit in your cohort from the internals using our FREE QCE Cohort Comparison Tool!
We’ve got plenty more practice questions for you to use to revise previous content from throughout the year! Check them out:
- Unit 3 Chemistry Data Test IA1 Practice Questions
- Multiple Choice Practice Questions for Unit 3 & 4 Chemistry External Assessment
- Download QCAA Chemistry Practice Exam for External Assessment Revision
You’ll also want to have a look at our nifty guides for working on your QCE Chemistry assessments below:
- The Definitive Guide to Writing a Student Experiment Report for QCAA Chemistry
- The Ultimate Guide to Conducting a QCAA Chemistry Research Investigation
- How to Ace Your External Assessment for QCAA Chemistry
- The Ultimate Guide to QCAA Chemistry Unit 3: Equilibrium, Acids and Redox Reactions
- The Ultimate Guide to QCAA Chemistry Unit 4: Structure, Synthesis and Design
Are you looking for some extra help with the QCAA Unit 3 & 4 Chemistry External Assessment?
We have an incredible team of QLD Chemistry tutors and mentors!
We can help you master the QCAA Chemistry syllabus and ace your upcoming Chemistry assessments with personalised lessons conducted one-on-one in your home or online!
We’ve supported over 8,000 students over the last 11 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational QLD tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Yalindi Binduhewa is an Art of Smart tutor based in Queensland and was part of the very first cohort to go through the ATAR system, so she knows exactly how fun and enjoyable it can be. She is currently studying a Bachelor of Medical Imaging (Honours) at QUT and is loving it. When she’s not doing uni-related stuff or tutoring, she’s hanging out with her friends, rewatching a show for the 100th time, or trying out new crafty projects and discovering that she doesn’t have a talent for everything.