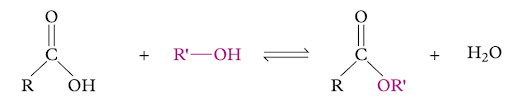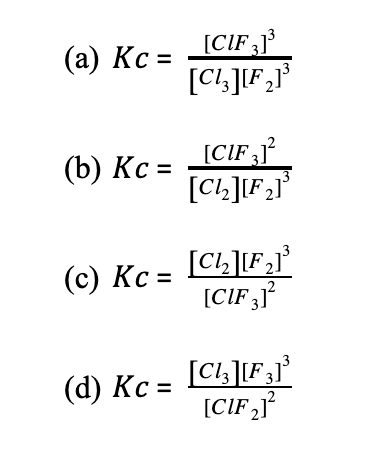Finding it difficult to come across multiple choice practice questions for the QCAA Chemistry External Assessment (EA)? Struggle no more, because we’ve got a bank of 25 questions to help you prep for your final Chemistry exam!
Once you’ve had a go at all 25 Chemistry EA multiple choice practice questions, you can go ahead and download the solutions to see how you’ve gone.
What are you waiting for? Let’s get to studying!
Chemistry EA Multiple Choice Practice Questions
Question 1
A tertiary carbon atom is
(a) bound to one other carbon
(b) bound to two other carbons
(c) bound to three other carbons
(d) bound to no other carbons
Question 2
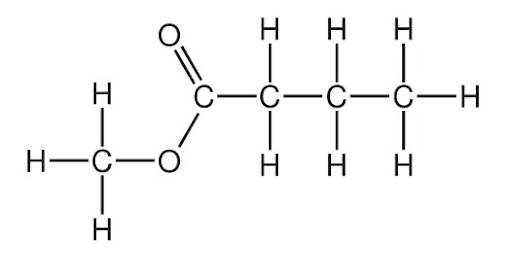
Use IUPAC conventions to name the following structure.
Image sourced from Chemistry Solutions
(a) methyl pentanoate
(b) butanoic acid
(c) propyl butanoate
(d) methyl butanoate
Question 3
Deduce what type of reaction the example below demonstrates.
Image sourced from Oxford Chemistry for Queensland Units 3 & 4
(a) substitution
(b) reduction
(c) esterification
(d) elimination
Question 4
Identify the reductant in the following redox reaction.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
(a) CuSO4(aq)
(b) Cu(s)
(c) Zn(s)
(d) ZnSO4(aq)
Question 5
Deduce the oxidation state of chlorine in HClO3.
(a) +5
(b) +4
(c) -5
(d) -4
Question 6
According to the reactivity series in the Formula and data book, determine the correct order for the following metals from most reactive to least reactive.
(a) Ni, Li, Fe
(b) Bi, Cu, Ca
(c) Al, Mn, Co
(d) Au, Ag, Ni
Question 7
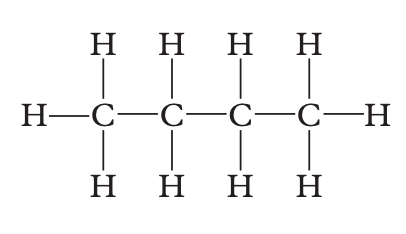
Determine which of the following is an isomer of the structure shown below.
Image sourced from Oxford Chemistry for Queensland Units 3 & 4
(a) propane
(b) 2-methylpropane
(c) butane
(d) 2-methylbutane
Question 8
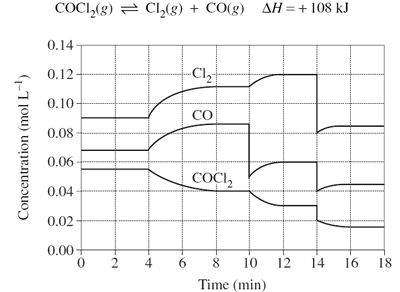
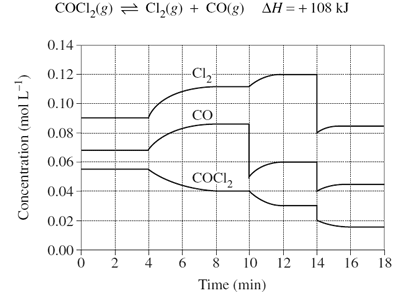
Predict the effect that increasing volume at time = 18mins will have on the reaction below.
Image sourced from Dynamic Science
(a) The system will partially oppose the change by moving to the product side to decrease the reactant concentration, resulting in a net forward reaction
(b) The system will partially oppose the change by moving to the reactant side to decrease the reactant concentration, resulting in a net forward reaction
(c) The system will partially oppose the change by moving to the product side to decrease the reactant concentration, resulting in a net reverse reaction
(d) The system will partially oppose the change by moving to the reactant side to decrease the product concentration, resulting in a net reverse reaction
Question 9
Determine the E° value of a Mg2+/Mg and Cu2+/Cu galvanic cell.
(a) 2.71V
(b) 2.22V
(c) 2.4V
(d) 2.7V
Question 10
Predict the effect that adding a catalyst at time = 5 mins would have had on the reaction below.
Image sourced from Dynamic Science
(a) The system will partially oppose the change by moving to the product side to decrease the reactant concentration, resulting in a net forward reaction
(b) No effect on equilibrium
(c) The system will partially oppose the change by moving to the product side to decrease the reactant concentration, resulting in a net reverse reaction
(d) Both the forward and reverse reactions will speed up equally
Question 11
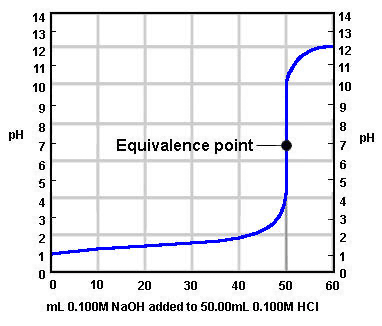
The graph below shows the titration between a strong acid and a strong base. What was the initial concentration of H+ ions in the acid?
Image sourced from Chem.ubc.ca
(a) 0.1
(b) 0.01
(c) 0.001
(d) 1
Question 12
Which of the options below correctly demonstrates oxidation of a primary alcohol?
(a) alcohol → ketone
(b) alcohol → aldehyde → carboxylic acid
(c) alcohol → carboxylic acid
(d) alcohol → ketone → carboxylic acid
Question 13
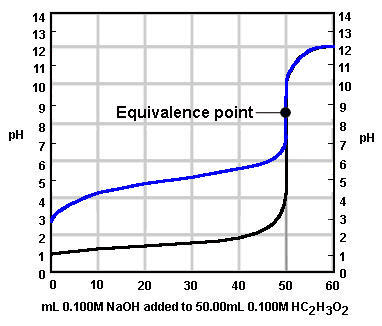
Which of the following pH indicators is most appropriate to use to detect the end point of the titration curve below?
Image sourced from Chem.ubc.ca
(a) Phenolphthalein
(b) Phenol Red
(c) Methyl Red
(d) Bromothymol Blue
Question 14
A galvanic electrochemical cell
(a) is non-spontaneous
(b) requires and external energy source
(c) converts chemical energy into electrical energy
(d) anode is positive and cathode is negative
Question 15
Deduce which of the following is a diprotic acid.
(a) H2SO4
(b) HNO3
(c) CH3COOH
(d) HCl
Question 16
Determine the correct statement regarding equivalence point and end point.
(a) equivalence point occurs after the end point
(b) a pH indicator changes colour at the equivalence point
(c) the number of moles of titrant and analyte are equal at the equivalence point
(d) a pH indicator changes colour at the half- equivalence point
Question 17
At equilibrium, a 1.00 L vessel contains 0.04 mol of H2, 0.06 mol of I2, and 0.35 mol of HI. The system is represented by the equation below. Determine, to two decimal places, the equilibrium constant (Kc) for this reaction.
H2(g) + I2(g) ⇌ 2HI(g)
(a) Kc = 50.04
(b) Kc = 40.05
(c) Kc = 40.04
(d) Kc = 51.04
Question 18
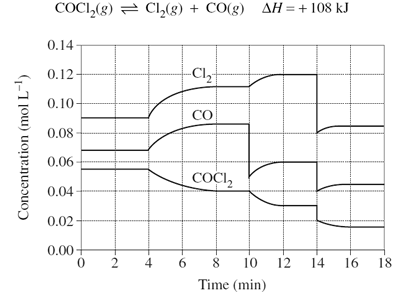
For the graph below, identify how many times the system equilibrium has been disturbed.
Image sourced from Dynamic Science
(a) 2
(b) 3
(c) 4
(d) It has not been disturbed
Question 19
Which of the following organic compounds contains a hydroxyl functional group?
(a) Alcohol
(b) Amide
(c) Nitrile
(d) Aldehyde
Question 20
What is the oxidation number of sulfur in H2SO4?
(a) -6
(b) +3
(c) -3
(d) +6
Question 21
The final shape of a protein molecule is known as its
(a) Primary structure
(b) Secondary structure
(c) Tertiary structure
(d) Final structure
Question 22
Determine the equilibrium constant for the following reaction.
2ClF3(g) ⇄ 3 F2(g) + Cl2(g)
Question 23
A condensation reaction is when
(a) Bonds are broken in double/triple bond of hydrocarbon and in covalent bond of second molecule
(b) One substituent on a saturated carbon chain is replaced by another substituent
(c) Substituents are removed from two adjacent carbon atoms in a haloalkane to form multiple bonds
(d) Two molecules combine to form a larger molecule and H2O
Question 22
Calculate the atom economy for H2O as the desired product in the following reaction.
CH4(g) + 2O2(g) → 2H2O(g) + CO2(g)
(a) 40%
(b) 4%
(c) 45%
(d) 50%
Question 25
Determine the order of the following intermolecular forces from strongest to weakest.
(a) hydrogen bonds, dipole-dipole interactions, dispersion forces
(b) dipole-dipole interactions, dispersion forces, hydrogen bonds
(c) hydrogen bonds, dispersion forces, dipole-dipole interactions
(d) none of the above
Solutions to Chemistry EA Multiple Choice Practice Questions
Click here to download the solutions to the 25 practice questions above!
Didn’t do so hot on that practice test? Don’t stress it! Recap on the essentials with our Unit 4 QCAA Chemistry Summary!
On the hunt for other QCAA Chemistry resources?
We’ve got plenty more practice questions for you to use to revise previous content from throughout the year! Check them out:
- Unit 3 Chemistry Data Test IA1 Practice Questions
- Practice Questions for Unit 3 & 4 Chemistry External Assessment
- Download QCAA Chemistry Practice Exam for External Assessment Revision
You’ll also want to have a look at our nifty guides for working on your QCAA Chemistry assessments below:
- The Definitive Guide to Writing a Student Experiment Report for QCAA Chemistry
- The Ultimate Guide to Conducting a QCAA Chemistry Research Investigation
- How to Ace Your External Assessment for QCAA Chemistry
- The Ultimate Guide to QCAA Chemistry Unit 3: Equilibrium, Acids and Redox Reactions
Are you looking for some extra help with the QCAA Unit 3 & 4 Chemistry External Assessment?
We have an incredible team of QLD Chemistry tutors and mentors!
We can help you master the QCAA Chemistry syllabus and ace your upcoming Chemistry assessments with personalised lessons conducted one-on-one in your home or online!
We’ve supported over 8,000 students over the last 11 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational QLD tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Yalindi Binduhewa is an Art of Smart tutor based in Queensland and was part of the very first cohort to go through the ATAR system, so she knows exactly how fun and enjoyable it can be. She is currently studying a Bachelor of Medical Imaging (Honours) at QUT and is loving it. When she’s not doing uni-related stuff or tutoring, she’s hanging out with her friends, rewatching a show for the 100th time, or trying out new crafty projects and discovering that she doesn’t have a talent for everything.