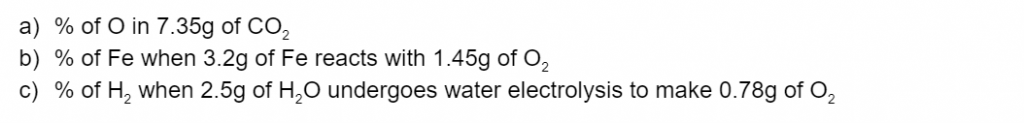If you’re looking to prepare yourself for Year 11 Chemistry Module 1: Properties and Structure of Matter with some practice questions, you’ve come to the right place!
We understand that in order to kick-butt in chemistry, we gotta master the basics first.
So, we’ve made and gathered 20 practice questions that address each of the 14 learning dot points of Properties and Structure of Matter.
Need to revise Module 1: Properties and Structure of Matter first? Check out our break down of the module in our guide here!
What are you waiting for? Let’s get down to it!
Properties of Matter
Inquiry Question: How do the properties of substances help us to classify and separate them?
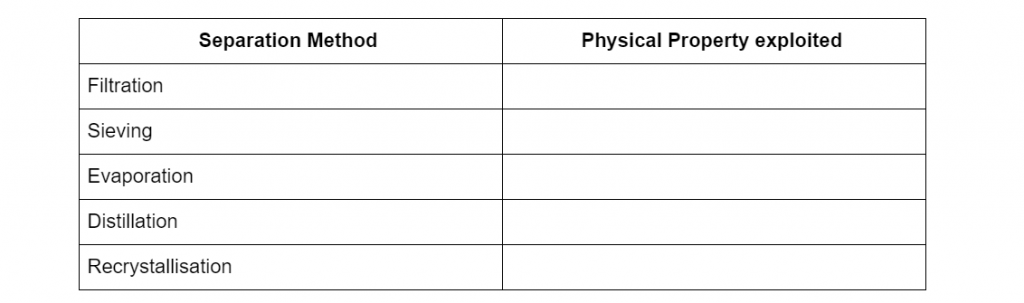
Question 1
Complete the following table of separation methods with the physical properties it uses to separate mixtures. (5 marks)
(L1.1: explore homogeneous mixtures and heterogeneous mixtures through practical investigations: using separation techniques based on physical properties)
Question 2
Draw a flow diagram of how you would separate a mixture of ethanol, water, salt and sand. (4 marks)
(L1.1: explore homogeneous mixtures and heterogeneous mixtures through practical investigations: using separation techniques based on physical properties)
Question 3
Calculate the percentage composition by weight of the following elements/compounds .(3 marks)
(L1.1: explore homogeneous mixtures and heterogeneous mixtures through practical investigations calculating percentage composition by weight of component elements and/or compounds)
Question 4
Name the following compounds. (8 marks)
(L1.2: investigate the nomenclature of inorganic substances using International Union of Pure and Applied Chemistry (IUPAC) naming conventions)
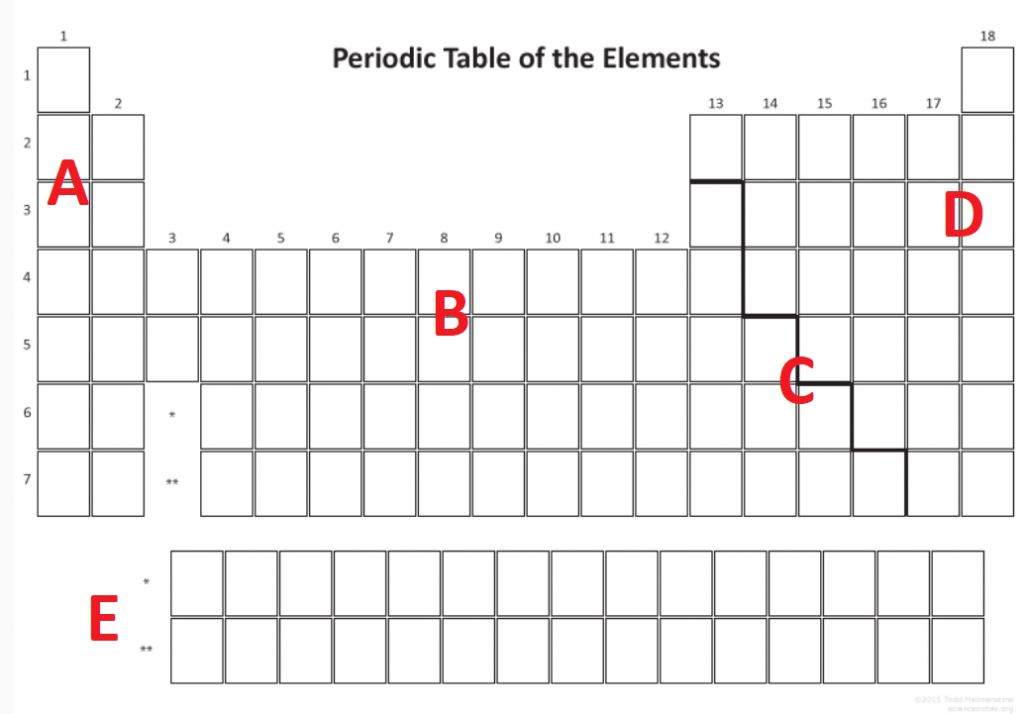
Question 5
Image sourced from Science Notes
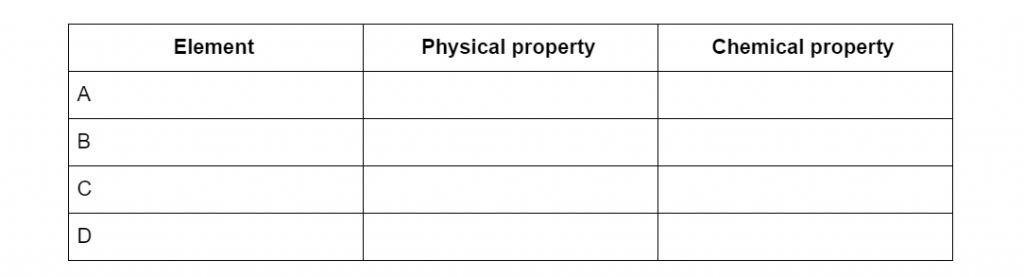
Using the periodic table in Question 5, list at least 2 physical and 2 chemical characteristics of element A, B, C and D in the table below:
(L1.3: classify the elements based on their properties and position in the periodic table through their physical properties and chemical properties)
Atomic Structure and Atomic Mass
Inquiry Question: Why are atoms of elements different from one another?
Question 6
With reference to the molecular structure of elements, explain the trends in reactivity of the elements as you. (4 marks)
a) Move across the periodic table
b) Move down the periodic table
(L2.1: investigate the basic structure of stable and unstable isotopes by examining their position in the periodic table, the distribution of electrons, protons and neutrons in the atom and representation of the symbol, atomic number and mass number (nucleon number))
Question 7
Explain how the composition of electrons, protons and neutrons in the atom contribute to the stability of the isotope. (3 marks)
(L2.1: investigate the basic structure of stable and unstable isotopes by examining their position in the periodic table, the distribution of electrons, protons and neutrons in the atom and representation of the symbol, atomic number and mass number (nucleon number))
Question 8
Determine the neutron to proton ratio (N:Z) of the following atoms and thus, its stability. (8 marks)
a) Ru-99
b) Pt-190
c) Ca-48
d) Kr-86
(L2.1: investigate the basic structure of stable and unstable isotopes by examining their position in the periodic table, the distribution of electrons, protons and neutrons in the atom and representation of the symbol, atomic number and mass number (nucleon number))
Question 9
Write the electronic configuration and the spdf notation for the following elements. (16 marks)
a) Lithium
b) Sodium
c) Sulfur
d) Oxygen
e) Carbon
f) Zinc
g) Iron
h) Neon
(L2.2: model the atom’s discrete energy levels, including electronic configuration and spdf notation)
If practice questions have you feeling overwhelmed, our patient Chemistry tutors near you can support you with tailored one on one tutoring in the comfort of your home or online.
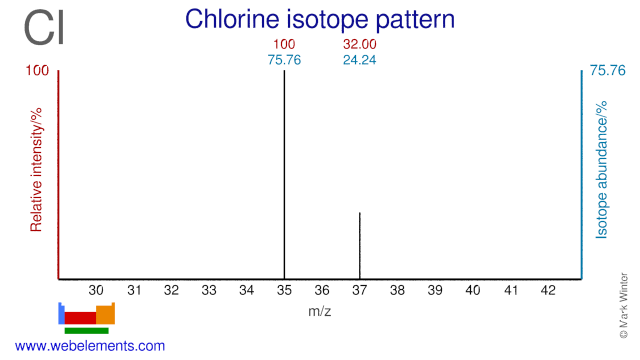
Question 10
Using the diagram below:
a) Determine the relative abundance of the two chlorine isotopes as percentages. (2 marks)
b) Determine the atomic weight of chlorine using the mass spectrum data. (2 marks)
Image sourced from Web Elements
(L2.3: calculate the relative atomic mass from isotopic composition)
Question 11
A student has found that sample A emitted blue/purple light while sample B emitted red light. Based on their observations, deduce what elements sample A or B might be and provide explanations for your choice (4 marks).
(L2.4: investigate energy levels in atoms and ions through collecting primary data from a flame test using different ionic solutions of metals)
Question 12
Assess the advantages and limitations of the Bohr model and the Schrödinger model as an account for spectral analysis. (6 marks)
(L2.4: investigate energy levels in atoms and ions through examining spectral evidence for the Bohr model and introducing the Schrödinger model)
Question 13
A scientist has found a new element, X and has derived the following to describe the new element:
Predict whether this new element is stable or unstable. Provide explanations (3 marks).
(L2.5: investigate the properties of unstable isotopes using natural and human-made radioisotopes as examples, including but not limited to types of radiation and types of balanced nuclear reactions)
Question 14
Write the nuclear decay equation that results in the following isotopes with their respective decay. (5 marks)
a) Th-234 (alpha decay)
b) U-234 (beta decay)
c) Ra-226 (alpha decay)
d) Xe-131 (beta decay)
(L2.5: investigate the properties of unstable isotopes using natural and human-made radioisotopes as examples, including but not limited to types of radiation and types of balanced nuclear reactions)
Periodicity:
Inquiry Question: Are there patterns in properties of elements?
Question 15
With reference to the periodic table, explain how trends in its periods and groups can be used to predict an element’s reactivity, electronegativity and state of matter at room temperature. (4 marks)
(L3.1: demonstrate, explain and predict the relationships in the observable trends in the physical and chemical properties of elements in periods and groups in the periodic table, including but not limited to the state of matter at room temperature, electronic configurations and atomic radii, first ionisation energy and electronegativity and reactivity with water)
Question 16
A scientist has found an element with a large atomic radii.
What implications does this have on its electronic configuration, reactivity with water and most probable state of matter at room temperature? Provide explanations. (6 marks)
(L3.1: demonstrate, explain and predict the relationships in the observable trends in the physical and chemical properties of elements in periods and groups in the periodic table, including but not limited to the state of matter at room temperature, electronic configurations and atomic radii, first ionisation energy and electronegativity and reactivity with water)
Question 17
Explain why the second ionization energy is lower than the first ionization energy with reference to the structure and composition of the element. (3 marks)
(L3.1: demonstrate, explain and predict the relationships in the observable trends in the physical and chemical properties of elements in periods and groups in the periodic table, including but not limited to the state of matter at room temperature, electronic configurations and atomic radii, first ionisation energy and electronegativity and reactivity with water)
Bonding
What binds atoms together in elements and compounds?
Question 18
Using their electronegativities, determine whether the following elements will form an ionic or covalent bond. Include calculations. (10 marks)
a) Sodium and fluoride
b) Magnesium and oxygen
c) Lead and chloride
d) Potassium and sulfur
e) Beryllium and Selenium
(L4.1: investigate the role of electronegativity in determining the ionic or covalent nature of bonds between atoms)
Question 19
Using Lewis dot diagrams, explain the differences between the ionic bond between NaCl and the covalent bond of H2O. (4 marks)
(L4.2: investigate the differences between ionic and covalent compounds through using nomenclature, valency and chemical formulae (including Lewis dot diagrams)
Question 20
Using named examples, explain how the electronegativity of atoms can be used to determine whether it will produce a polar compound. (3 marks)
(L4.2: investigate the differences between ionic and covalent compounds through examining the spectrum of bonds between atoms with varying degrees of polarity with respect to their constituent elements’ positions on the periodic table
Question 21
Illustrate why the shape of a water molecule is bent and not linear. Include diagrams. (3 marks)
(L4.2: investigate the differences between ionic and covalent compounds through modelling the shapes of molecular substances)
Question 22
Carbon has many allotropes. Discuss how the structure of 3 carbon allotropes contribute to their uses. (7 marks)
(L4.3: investigate elements that possess the physical property of allotropy)
Question 23
Account for the physical and chemical properties that result from the following chemical structures: (12 marks)
a) Ionic networks
b) Covalent networks
c) Covalent molecular
d) Metallic structure
(L4.4: investigate the different chemical structures of atoms and elements, including but not limited to ionic networks, covalent networks (including diamond and silicon dioxide), covalent molecular and metallic structure)
Question 24
Explain how intramolecular and intermolecular bonds differ, with reference to its effect on the strength of a named compound (4 marks).
Question 25
Between HCl, H2O and H2, which substance would have the highest intermolecular strength? Include diagrams in your explanation. (6 marks)
(L4.5: explore the similarities and differences between the nature of intermolecular and intramolecular bonds and the strength of the forces associated with each, in order to explain the physical properties of elements and physical properties of compounds)
And that wraps up our 25 practice questions Year 11 Chemistry Module 1: Properties and Structure of Matter — good luck!
You can have a go at our practice questions for other modules below:
- Module 2: Introduction to Quantitative Chemistry Practice Questions
- 20 Practice Questions for Module 3: Reactive Chemistry
- 20 Practice Questions for Module 4: Drivers of Reactions
Aiming for a Band 6 in HSC Chemistry? Check out our guide to scoring one here!
Looking for some extra help with Properties and Structure of Matter?
We have an incredible team of Year 11 Chemistry tutors and mentors who are new HSC syllabus experts!
We can help you master Year 11 Chemistry Module 1: Properties and Structure of Matter and ace your upcoming Year 11 Chemistry assessments with personalised lessons conducted one-on-one in your home or at one of our state of the art campuses in Hornsby or the Hills!
We’ve supported over 8,000 students over the last 10 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational Year 11 Chemistry tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.