Can’t seem to find practice questions for Year 11 Chemistry Module 3: Reactive Chemistry?
We’ve got your back!
We totally understand how difficult it can be to find Year 11 practice questions online, especially ones that correlate with the new HSC syllabus.
To help you, we’ve selected and composed 20 practice questions that address each of the inquiry questions in Chemistry Module 3: Reactive Chemistry.
So, what are you waiting for? It’s time to consolidate your knowledge of Reactive Chemistry!
Chemical Reactions
Predicting Reactions of Metals
Rates of Reactions
Chemical Reactions
Inquiry Question 1: What are the products of a chemical reaction?
Question 1
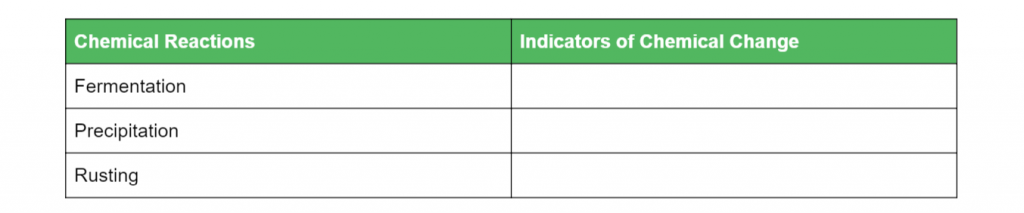
Complete the table of chemical reactions below with their respective indicators of chemical change: (6 marks)
L1.1: Investigate a variety of reactions to identify possible indicators of a chemical change
Question 2
Draw a diagram depicting how atoms are rearranged to form new substances during the reaction of an active metal, Mg with water. Label the reactants and products. (4 marks)
(L1.2: Use modelling to demonstrate: – the rearrangement of atoms to form new substances – the conservation of atoms in a chemical reaction)
Question 3
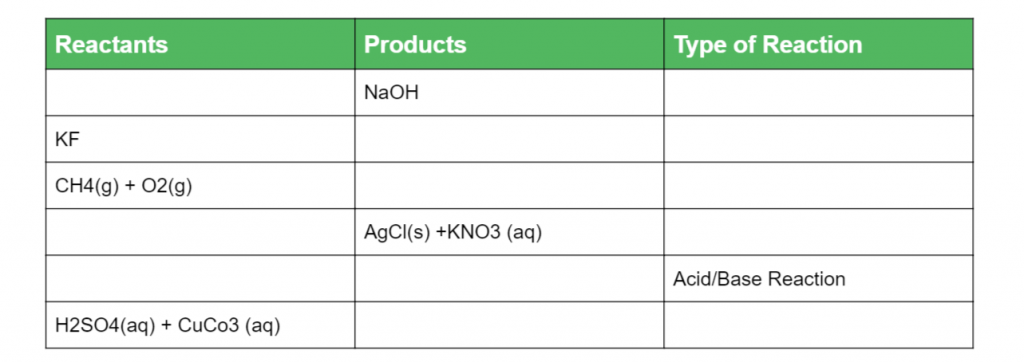
Complete the following table below: (6 marks)
(L1.3: Conduct investigations to predict and identify the products of a range of reactions, for example: Synthesis, Decomposition, Combustion, Precipitation, Acid/Base reactions, Acid/ carbonate reactions)
Question 4
Explain how Aboriginal and Torres Strait Islander People detoxify poisonous foods with a specific food example. (4 marks)
L1.4: Investigate the chemical processes that occur when Aboriginal and Torres Strait Islander Peoples detoxify poisonous food items
Question 5
Write the correct balanced equations for the following reactions (5 marks):
a) The combustion of butane
b) Reaction between calcium carbonate and nitric acid
c) The precipitation of lead iodide
d) The decomposition of copper fluoride
e) The synthesis of sodium carbonate
L1.5: Construct balanced equations to represent chemical reactions
Predicting Reactions of Metals
Inquiry Question 2: How is the reactivity of various metals predicted?
Question 6
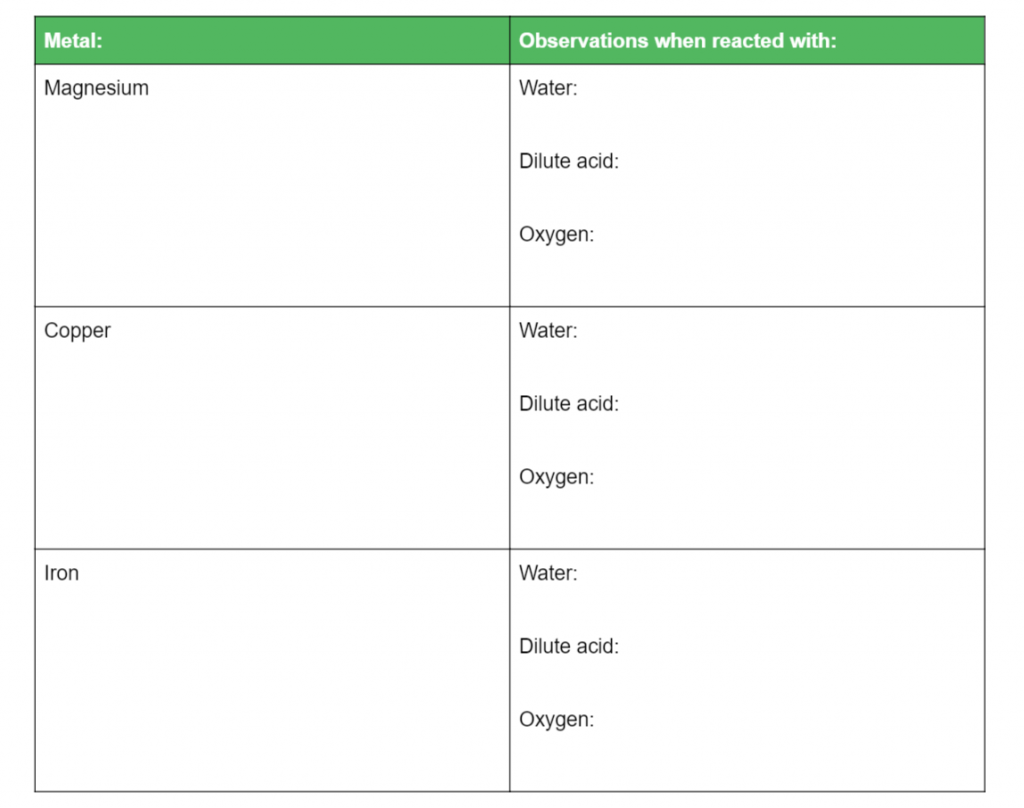
Complete the table below and then rank the metals based on their reactivity: (8 marks)
L2.1:Conduct practical investigations to compare the reactivity of a variety of metals in: Water, Dilute acid, Oxygen, Other metal ions in solution
Question 7
Compare the similarities and differences between your results from your metal activity series practical from the theoretical metal activity series from secondary sources (2 marks).
L2.2: construct a metal activity series using the data obtained from practical investigations and compare this series with that obtained from standard secondary-sourced information
Question 8
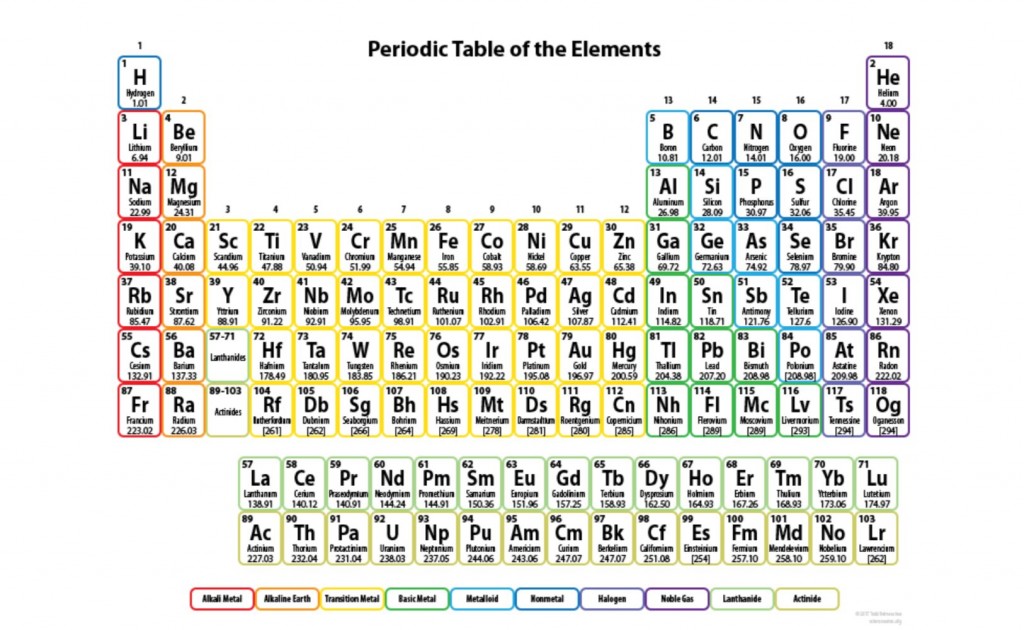
Label the ionization energy, atomic radius and electronegativity patterns in the periodic table below: (3 marks)
Image sourced from Science Notes
Question 9
Describe and explain the trends in metal activity on the periodic table in terms of their ionization energy, atomic radius and electronegativity. (6 marks)
L2.3: analyse patterns in metal activity on the periodic table and explain why they correlate with, for example: ionisation energy, atomic radius, electronegativity
Question 10
Identify which is the reductant in the following reaction (3 marks):
a) CH4(g) + O2 (g) → H2O(l) + CO2 (g)
b) Fe3O4(aq) + 4H2 (g) → 3Fe(aq) + 4H2O (l)
c) 4Al(s) +3O2(g) → 2Al2O3(s)
L2.4: apply the definitions of oxidation and reduction in terms of electron transfer and oxidation numbers to a range of reduction and oxidation (redox) reactions
Question 11
Describe how you would measure and compare the reduction potential of galvanic half-cells containing copper and zinc. Include diagrams if necessary. (6 marks)
L2.5: conduct investigations to measure and compare the reduction potential of galvanic half-cells
Question 12
Write the half -equations and balanced overall redox equations for the reactions of the following metals:
a) Copper and iron
b) Silver and lead
c) Nickel and Magnesium
L2.6: Construct relevant half-equations and balanced overall equations to represent a range of redox reactions
Question 13
Following from Question 11, calculate the cell potential for each of the metal pairs (3 marks)
L2.6: Construct relevant half-equations and balanced overall equations to represent a range of redox reactions
Question 14
Identify the oxidant and reductant between the following metal pairs: (5 marks)
a) Magnesium and Barium
b) Mercury and Iron
c) Nickel and Silver
d) Gold and Lead
e) Copper and Tin
L2.7: predict the reaction of metals in solutions using the table of standard reduction potentials
Question 15
Explain how to predict whether a redox reaction is spontaneous or not using cell potentials (3 marks).
L2.8: predict the spontaneity of redox reactions using the value of cell potentials (ACSCH079, ACSCH080
Rates of Reactions
Inquiry Question 3: What affects the rate of a chemical reaction?
Question 16
Explain how the following factors can influence the rate of reaction: (5 marks)
a) Increasing temperature on an endothermic reaction
b) Decreasing temperature on an exothermic reaction
c) Using a powdered reagent as opposed to a massive block of reagent
d) Increasing the concentration of reactant
e) The addition of catalysts to an exothermic reaction
L3.1: conduct a practical investigation, using appropriate tools (including digital technologies), to collect data, analyse and report on how the rate of a chemical reaction can be affected by a range of factors, including but not limited to: temperature, surface area of reactant(s), concentration of reactant(s), catalysts (ACSCH042)
Question 17
A student grounded a block of reagent to powder to influence its rate of reaction. Explain how using a powdered reagent is more preferable to using a massive block of reagent (3 marks).
Question 18
Explain the role of a catalyst in altering the rate of reaction. (3 marks)
L3.1 Conduct a practical investigation, using appropriate tools (including digital technologies), to collect data, analyse and report on how the rate of a chemical reaction can be affected by a range of factors, including but not limited to: temperature, surface area of reactant(s), concentration of reactant(s), catalysts (ACSCH042).
Question 19
Explain how molecular orientation affects activation energy, with reference to the collision theory (4 marks).
L3.2: investigate the role of activation energy, collisions and molecular orientation in collision theory
Question 20
With reference to the collision theory, explain how temperature changes can alter reaction rate (4 marks).
L3.2: explain a change in reaction rate using collision theory
And that wraps up our 20 practice questions for Year 11 Chemistry Module 3: Reactive Chemistry – good luck!
You can have a go at our practice questions for other modules below:
- Practice Questions for Module 1: Properties and Structure of Matter
- Module 2: Introduction to Quantitative Chemistry Practice Questions
- 20 Practice Questions for Module 4: Drivers of Reactions
Aiming for a Band 6 in HSC Chemistry? Check out our guide here to scoring one!
Need some extra help with Reactive Chemistry?
We have an incredible team of Year 11 Chemistry tutors and mentors who can provide live online tutoring or Chemistry tutoring near you.
We pair you with a fantastic tutor who will tutor you via video with an interactive whiteboard PLUS give you digital access to our world class resources so that you’re well prepared!
We’ve supported over 8,000 students over the last 10 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational HSC tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.