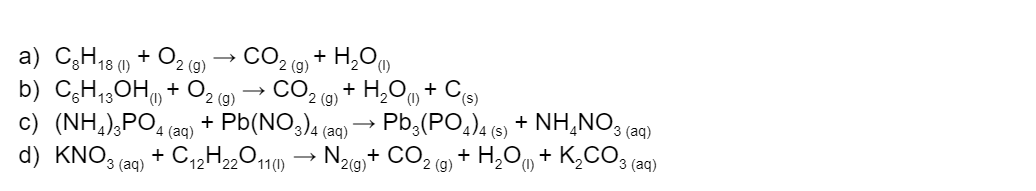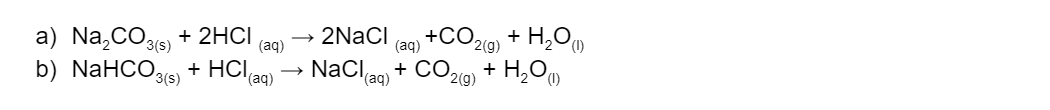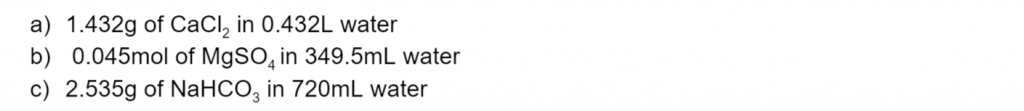“Practice makes perfect – but where can I find more questions on the Year 11 Chemistry Module 2: Introduction to Quantitative Chemistry?”
Lucky for you, we have created and compiled 20 practice questions that address each of the 9 dot points of Introduction to Quantitative Chemistry.
If you need a quick recap of Module 2: Introduction to Quantitative Chemistry, make sure you check out our break down guide here!
So what are you waiting for? Let’s get started!
Chemical Reactions and Stoichiometry
Question 1
Following gravimetric analysis, you have found that your experimental mass value is lower than the theoretical mass. Account for this difference with two explanations (3 marks).
(L1.1: Conduct practical investigations to observe and measure the quantitative relationships of chemical reactions, including but not limited to masses of solids and/or liquids in chemical reaction and volumes of gases in chemical reactions)
Question 2
If one of the products of a chemical reaction is a gas, the total volume that the atoms occupy increases after a reaction. Is this true? Explain why or why not. (3 marks)
(L1.1: Conduct practical investigations to observe and measure the quantitative relationships of chemical reactions, including but not limited to masses of solids and/or liquids in chemical reaction and volumes of gases in chemical reactions)
Question 3
Explain how you would investigate the Law of Conservation through an experiment that involves quantifying the volumes of gases in chemical reactions. (4 marks)
(L1.1: Conduct practical investigations to observe and measure the quantitative relationships of chemical reactions, including but not limited to masses of solids and/or liquids in chemical reaction and volumes of gases in chemical reactions)
Question 4
Balance the following equations. (4 marks)
(L1.2: Relate stoichiometry to the law of conservation of mass in chemical reactions by investigating balancing chemical equations (ACSCH039) and solving problems regarding mass changes in chemical reactions (ACSCH046))
If you want to conquer the Chemistry syllabus and ensure you have a strong understanding of each topic, our passionate Chemistry tutors near you are ready to help!
Question 5
A student combines 154 grams of carbon tetrachloride and an unknown quantity of bromine in a sealed container to produce 243 grams of dibromodichlormethane and 71 grams of chlorine. How much chlorine was used in the reaction, assuming the reactants are completely used up? (3 marks)
Question sourced from sciencing
(L1.2: Relate stoichiometry to the law of conservation of mass in chemical reactions by investigating balancing chemical equations and solving problems regarding mass changes in chemical reactions)
Mole Concept
Question 6
Design an experiment that aims to obtain the molar mass of magnesium. (6 marks)
(L2.1: conduct a practical investigation to demonstrate and calculate the molar mass (mass of one mole) of an element, a compound)
Question 7
A sample of 0.42mg CO2 gas was found to occupy 3.65L of volume. Explain how the “Ideal Gas Law” can be used to obtain the molar mass of CO2. Calculate the molar mass of CO2. (4 marks)
(L2.1: conduct a practical investigation to demonstrate and calculate the molar mass (mass of one mole) of an element, a compound)
Question 8
Design an experiment that tests whether the following reactions carry on in simple whole number ratios. (6 marks)
(L2.2: conduct an investigation to determine that chemicals react in simple whole number ratios by moles)
Question 9
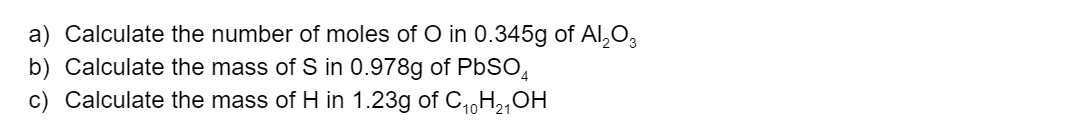
Answer the following questions: (6 marks)
(L2.3: explore the concept of the mole and relate this to Avogadro’s constant to describe, calculate and manipulate masses, chemical amounts and numbers of particles in: moles of elements and compounds ? = ?/Mm (n = chemical amount in moles, m = mass in grams, ?/m= molar mass in gmol-1)
Question 10
Polymers are large molecules composed of simple units repeated many times. Thus, they often have relatively simple empirical formulas. Calculate the empirical formulas of the following polymers. (5 marks)
a) Lucite (Plexiglas); 59.9% C, 8.06% H, 32.0% O
b) Saran; 24.8% C, 2.0% H, 73.1% Cl
c) polyethylene; 86% C, 14% H
d) polystyrene; 92.3% C, 7.7% H
e) Orlon; 67.9% C, 5.70% H, 26.4% N
Question sourced from Chemistry Libretexts
(L2.3: Explore the concept of the mole and relate this to Avogadro’s constant to describe, calculate and manipulate masses, chemical amounts and numbers of particles in percentage composition calculations and empirical formulae)
Question 11
Souring of wine occurs when ethanol is converted to acetic acid by oxygen by the following reaction:
A 1.00 L bottle of wine, labeled as 8.5% (by volume) ethanol, is found to have a defective seal. Analysis of 1.00 mL showed that there were 0.0274 grams of acetic acid in that 1.00 mL. The density of ethanol is 0.816 g/mL and the density of water is 1.00 g/mL.
a) What mass of oxygen must have leaked into the bottle? (3 marks)
b) What is the percent yield for the conversion of ethanol to acetic acid if O2 is in excess? (4 marks)
Question sourced from Washington University in St. Louis Department of Chemistry
(L2.3: explore the concept of the mole and relate this to Avogadro’s constant to describe, calculate and manipulate masses, chemical amounts and numbers of particles in limiting reagent reactions)
Concentration and Molarity
Question 12
Explain how you have used gravimetric analysis to find the concentration of an unknown solution. (3 marks)
(L3.1: conduct practical investigations to determine the concentrations of solutions and investigate the different ways in which concentrations are measured)
Question 13
During gravimetric analysis, the experimental value was found to be less than the theoretical concentration of the solution. Explain THREE sources of error and suggest ways to prevent them (3 marks).
(L3.1: conduct practical investigations to determine the concentrations of solutions and investigate the different ways in which concentrations are measured)
Question 14
Calculate the concentration (in mol/L) of the following (6 marks):
Question 15
What is the molar concentration of chloride ions in a solution prepared by mixing 100.0 mL of 2.0 M KCl with 50.0 mL of a 1.50 M CaCl2 solution? (5 marks)
(L3.2: manipulate variables and solve problems to calculate concentration, mass or volume using ? = ?/? (molarity formula), dilutions (number of moles before dilution = number of moles of sample after dilution))
Question 16
Describe how you would make a standard solution of 0.1M hydrochloric acid and dilute it to 0.02M. Include relevant calculations and safety precautions. (7 marks)
(L3.3: conduct an investigation to make a standard solution and perform a dilution)
Gas Laws
Question 17
A student hypothesized that N2 gas in the balloon would deflate upon increased temperature. Will his hypothesis be proven correct or wrong? Explain your answer with reference to Gay-Lussac’s Law and the Ideal Gas Law (4 marks).
(L4.1: conduct investigations and solve problems to determine the relationship between the Ideal Gas Law and Gay-Lussac’s Law (temperature)
Question 18
With reference to Boyle’s law, explain the effect of increasing pressure on the volume of a known gas with a constant mass and temperature. How is this represented in the Ideal Gas Law equation? (4 marks)
(L4.1: conduct investigations and solve problems to determine the relationship between the Ideal Gas Law and Boyle’s Law)
Question 19
Using the Charles Law, explain the effect of increasing temperature on the molecular characteristics of gases. How is this represented in the Ideal Gas Law equation? (3 marks).
(L4.1: conduct investigations and solve problems to determine the relationship between the Ideal Gas Law and Charles’ Law)
Question 20
During an experiment, a student has found that the volume and amount of moles of O2 gas are not proportional to each other. Using the Avogadro’s Law, explain any sources of error and suggest ways to avoid them (4 marks).
(L4.1: conduct investigations and solve problems to determine the relationship between the Ideal Gas Law and Avogadro’s Law)
And that wraps up our 20 practice questions for Year 11 Chemistry Module 2: Introduction to Quantitative Chemistry – good luck!
You can have a go at our practice questions for other modules below:
- Practice Questions for Module 1: Properties and Structure of Matter
- 20 Practice Questions for Module 3: Reactive Chemistry
- 20 Practice Questions for Module 4: Drivers of Reactions
Aiming for a Band 6 in HSC Chemistry? Check out our guide to scoring one here!
Looking for some extra help with Introduction to Quantitative Chemistry?
We have an incredible team of Year 11 Chemistry tutors and mentors who are new HSC syllabus experts!
We can help you master Introduction to Quantitative Chemistry and ace your upcoming Chemistry assessments with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby!
We’ve supported over 8,000 students over the last 10 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational Year 11 Chemistry tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.