How should you react to Year 11 Chemistry Module 3: Reactive Chemistry?
Just like the periodic table itself, there’s a variety of possible ways to react!
To the untrained eye, the periodic table just looks like an inconveniently-shaped table… and maybe sometimes the instructions telling you how to build it are as mysterious as IKEA’s.
To the eye in-training, this thing (the Periodic Table) is the centre-of-operations at the construction site of your chemical understanding. It’s the solid foundation that all your future chemistry mastery is going to spring upwards out of.
The periodic table is the centre of Chemistry as a subject and in particular, the Reactive Chemistry module.
By completing Year 11 Chemistry Module 1 and 2, you should now understand as much as possible about the individual building blocks the Universe is built out of.
And now it’s time for the next phase in the revised Chemistry syllabus — to start throwing these things together and see what they do!
In this article we’ll cover the content changes, a breakdown of Module 3: Reactive Chemistry and it’s inquiry questions as well as how to get a Band 6 in this unit!
What are the general changes to Year 11 Chemistry?
Overview of Module 3: Reactive Chemistry
How to Get a Band 6 in Module 3: Reactive Chemistry
What are the general changes to Year 11 Chemistry?
The major shift is depth-over-breadth, strongly focused in on core/pillar ideas.
Depth-exploring the ideas threaded into all of chemistry gives you a more integrated understanding and better matches what’s needed to make waves at Uni.
This means you have to be more active in your study (being tested on deep understanding of core concepts)
It also means you need to carry the understanding developed in year 11 forwards to year 12. In year 11 you’re putting your year 12 toolbox together. If you’re serious about knocking your band 6 out of the park next year, master year 11. The new syllabus is deliberately designed to always build upwards; and you want to be building on the strongest foundations and framework next year!
So, don’t overreact!
Overview of Module 3: Reactive Chemistry
Inquiry Question 1: What are the products of a chemical reaction?
At the end of the day, this is what chemistry does, as a power that sculpts this world:
- Grab some bagfuls of molecules (molecules are just atoms grouped in to temporary clusters) and bring them all together on the same playground (usually solvent, often air, sometimes combinations with solids).
- Let the molecules smash together, trade atoms (transact electrons in/out of their valence shells) and reorganise themselves in to different groupings until everything settles down in to a new status-quo.That’s basically what chemistry does.
Your periodic table gives you all the bond-swapping behaviours (electron shedding, electron snatching and electron sharing) when valence shells come together.
In this section, you’ll be looking at the variety of different things that can happen when molecules come together:
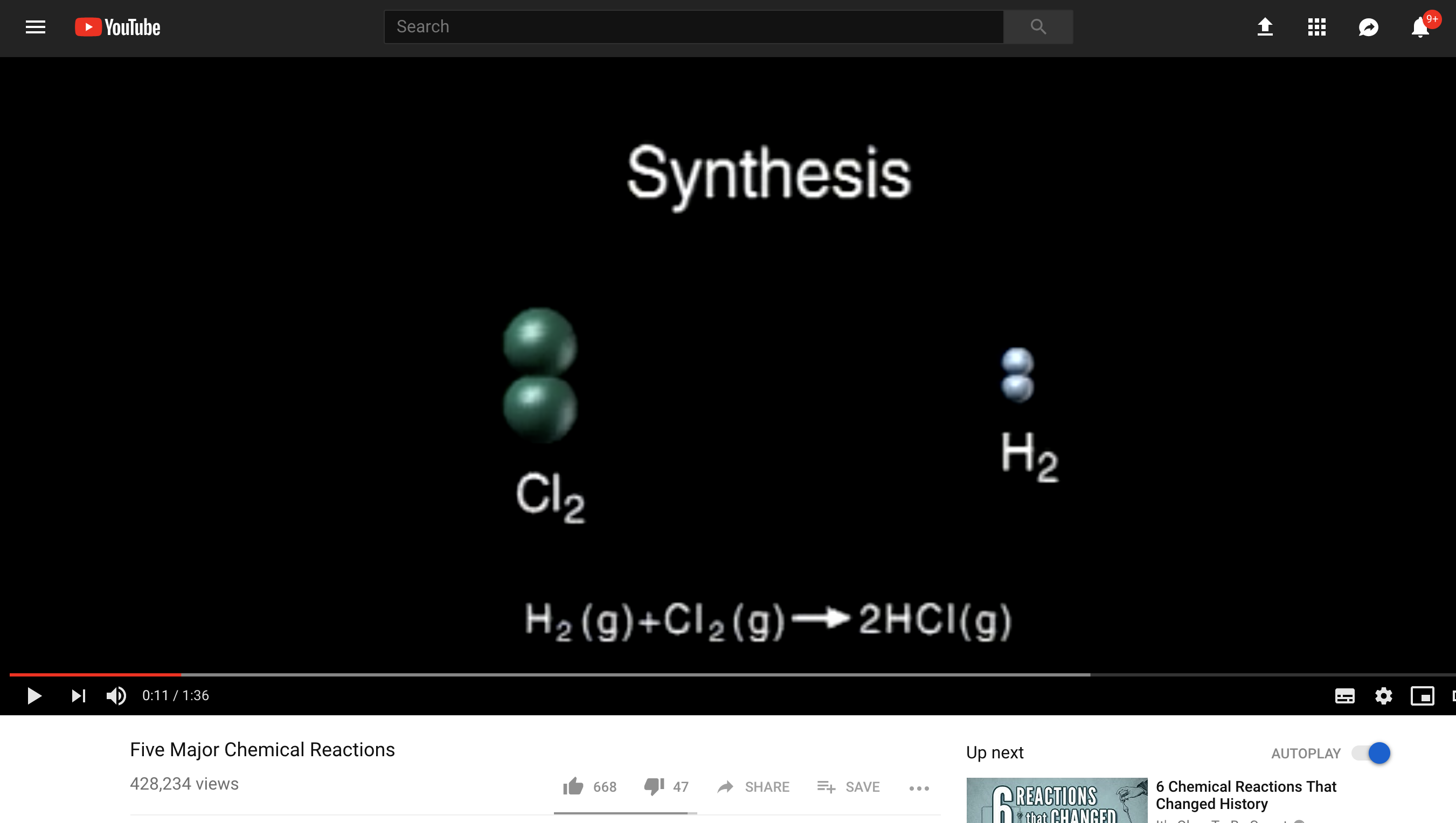
- Synthesis: we can join molecules up in to bigger structures; think photosynthesis or Panadol synthesis – also called addition
- Decomposition: we can take bigger molecules and bump them so they fall apart
- Combustion: reacting molecules (fuels) with oxygen to make oxides, like CO2; usually spilling energy out of bonds and in to the world as heat/pressure
- Precipitation: when ions wading through solvent find each other and fall in to an orderly seating arrangement, like people in the mall when the movie’s about to start at the cinema. The solid crystals are called a precipitate because they seem to be materialising out of nowhere like clouds/rain.
- Acid/base reactions what happens when the most common element in the universe (H) is dangling off a molecule by the skin of its teeth?
As well as the balancing-act skill that is balancing chemical equations (chemistry doesn’t lose atoms; it takes what’s there and re-arranges them in to transient formations)
Our experienced Chemistry tutors near you can support you one on one with consolidating your Year 11 Chemistry knowledge in the comfort of your own home or online.
Inquiry Question 2 : How is the reactivity of various metals predicted?
We’re on the left side of the Periodic Table.
These guys do everything they do by forcing electrons on the rest of the table around.
This is the first real chance you get to see how reaction behaviour bursts straight out of everything you’ve learned about periodic table position. You better believe this is all going to be about how electrons fall off metals as soon as water, acid or oxygen wander past to collect them (as expected).
Inquiry Question 3: What affects the rate of a chemical reaction?
So, there’s your bicycle rusting… and trees growing out of the air – literally! Trees add most of their bulk weight over time by plucking CO2 out of the air and spitting the O2 back out (thank you trees!).
Then there’s the chemical reaction between the Hindenburg’s gaseous contents and the rest of the air around it:
Some groups of molecules do their bond-swapping at such a creeping snail’s pace that it’s imperceptible.
Others smash their way to the end of their chemical activities like meteorites.
In this section you will zoom all the way down to that concert of molecules and see what’s really going on.
How to Get a Band 6 in Module 3: Reactive Chemistry
Tip #1: Keep adding connections to your giant wall periodic table
The periodic table is the entire centrepiece for this subject. It is the ultimate mind-map.
Print off the biggest periodic table you can (try the library or Officeworks) and just keep piling understanding and connections on top of it.
In particular, you’re going to be adding metal reactivity on the left side. It relates directly to what should still be bolted on to your periodic table’s left side (valence shells, atomic radii, electronegativity, ionisation energy).
By the time you get to the end of your 12, your periodic table should be covered with your entire conceptual understanding mapped out.
Tip #2: Always look at Reactive Chemistry through three lenses
Research in chemistry education clearly shows that students who well and truly lock in their Band 6 reactions from a few viewpoints at the same time:
Whenever you write a reaction equation, for example this one:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Always, always, force yourself to remember two things:
Firstly, the written items are real objects clashing together like armies by the trillion (supposing we have a few grams of the stuff) and they’re clashing together gymnastically in a dynamic, interactive, unceasing concert.
Secondly, don’t lose sight of the fact that the letters (“N”, for example) are shorthand for all the things you’ve already learned about complicated electronic sub-structure
Then, when you are considering any reaction in chemistry ask yourself two questions:
Firstly, how do the things I observe make complete sense in terms of what individual molecules are doing as they zip around and clash together?
Secondly, how does the reaction that happens make complete sense in terms of the electronic structure on the periodic table?
Always imagine yourself surveying the reactions from the Magic Schoolbus when you write abstractions of the reactions with letters on paper.
If you can make your observations of reactions in the lab completely agree with the microscopic view AND the periodic table inter-relationships, you’re really on the road to mastering the core concepts that drive chemistry!
Tip #3: Rewatch the reactions you did at school on Youtube
Re-do your experiments from school with screen experiments at home
Once you’ve seen or performed the experiment in class, you want to watch them being performed on Youtube. This is important because you can watch them in slow motion and in high definition.
You should see the reactions in their atomic or molecular form:
You can find more molecular animations of chemical reactions at these sites:
Preparatory Chemistry
And here are some awesome screen experiments (or virtual simulations):
ACS: Chemistry for Life
ChemCollective
Teach Chemistry
Amrita Lab
Tip #4: Recast the role of class-time in your life
For the rest of your life, your main learning grounds will be outside of class. Class is a place where you have access to an expert consultant (your teacher!).
Think about the questions you have before class. Jump ahead through the textbook and repurpose your class as a revision/understanding-checking session.
The HSC is your company and you’re the CEO! So make the most of it!
On the hunt for other Year 11 Chemistry resources?
Check out our module guides below:
- Module 1: Structure and Properties of Matter
- Module 2: Quantitative Chemistry
- Module 4: Drivers of Reactions
We’ve also got other practice questions which you can try below:
- Properties and Structure of Matter Practice Questions
- Quantitative Chemistry Practice Questions
- Reactive Chemistry Practice Questions
- Drivers of Reactions Practice Questions
Looking for extra help with Year 11 Reactive Chemistry?
We pride ourselves on our inspirational HSC Chemistry coaches and mentors!
We offer tutoring and mentoring for Years K-12 in a variety of subjects, with personalised lessons conducted one-on-one in your home or at one of our state of the art campuses in Hornsby or the Hills!
Our support isn’t just limited to these campuses. If you’re looking for support elsewhere, we also have fantastic Western Sydney tutors who can help you achieve your academic goals.
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on TikTok!
Adrian Wendeborn is a qualified science and maths teacher with a physics/chemistry double-major degree from USYD and a GDipEd from UQ. Adrian has taught in QLD and NSW and has worked with Art of Smart Education as a campus teacher, tutor, resource developer and Head of Faculty.