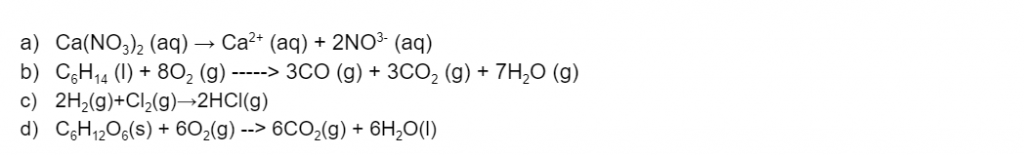Looking for some Year 11 Chemistry Module 4: Drivers of Reactions practice questions to get some practice under your belt?
You’re in luck! We’ve composed and compiled 20 practice questions that cover the 3 topics and all 13 learning dot points from Drivers of Reactions.
So what are you waiting for? Let’s jump into it!
Energy Changes in Chemical Reactions
Question 1
Identify TWO safety hazards that can occur when investigating an exothermic reaction and provide TWO ways to minimise this. (4 marks)
(L1.1:conduct practical investigations to measure temperature changes in examples of endothermic and exothermic reactions, including combustion)
Question 2
A student hypothesised that the dissociation of ionic substances are always exothermic. Design an experiment that tests this hypothesis with specific examples of ionic substances in aqueous solutions. (5 marks)
(L1.1:conduct practical investigations to measure temperature changes in examples of endothermic and exothermic reactions, including: dissociation of ionic substances in aqueous solution)
Question 3
1.80g of ethanol was burned in 220ml of water that increased from a temperature of 20°C to 68°C. Calculate the molar heat of combustion of ethanol. (3 marks)
(L1.2: investigate enthalpy changes in reactions using calorimetry and ? = ???? (heat capacity formula) to calculate, analyse and compare experimental results with reliable secondary-sourced data, and to explain any differences)
Question 4
a) How much heat is produced when 100 mL of 0.250 M HCl (density, 1.00 g/mL) and 200 mL of 0.150 M NaOH (density, 1.00 g/mL) are mixed?
b) If both solutions are at the same temperature and the heat capacity of the products is 4.19 J/g °C, how much will the temperature increase? What assumption did you make in your calculation?
Question sourced from Chemistry LibreTexts
(L1.2: investigate enthalpy changes in reactions using calorimetry and ? = ???? (heat capacity formula) to calculate, analyse and compare experimental results with reliable secondary-sourced data, and to explain any differences)
Need tutoring support? Our experienced Chemistry tutors near you will tailor their methods to your individual needs, helping you achieve both your academic and holistic goals.
Question 5
Construct energy profile diagrams of an exothermic and endothermic reaction to explain the thermodynamic differences between them. (4 marks)
L1.3: construct energy profile diagrams to represent and analyse the enthalpy changes and activation energy associated with a chemical reaction).
Question 6
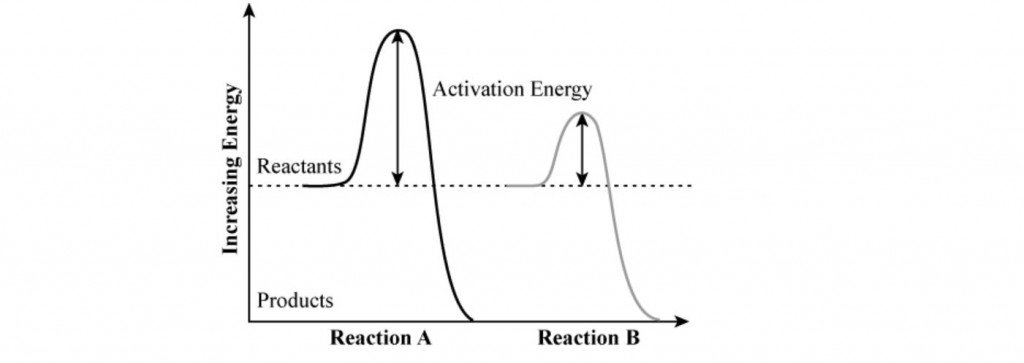
Considering Reaction A and Reaction B in the diagram below, explain the implications of its activation energy on its reactivity. (3 marks)
Image sourced from Chegg
(L1.3: construct energy profile diagrams to represent and analyse the enthalpy changes and activation energy associated with a chemical reaction)
Question 7
Using graphs, explain how a catalyst might affect a reaction. (3 marks)
(L1.4: model and analyse the role of catalysts in reactions)
Enthalpy and Hess’s Law
Question 8
With reference to the law of conservation of energy, explain why the dissociation of ionic substances in aqueous solutions are always endothermic on a molecular level (4 marks)
(L2.1: explain the enthalpy changes in a reaction in terms of breaking and reforming bonds, and relate this to the law of conservation of energy)
Question 9
With reference to the law of conservation of energy, explain the enthalpy changes for the following reactions. (3 marks)
(L2.1: explain the enthalpy changes in a reaction in terms of breaking and reforming bonds, and relate this to the law of conservation of energy)
Question 10
Draw an enthalpy diagram to illustrate the formation of carbon dioxide, showing both the direct path and indirect path with arrows labelled with its enthalpies. (3 marks)
(L2.2: investigate Hess’s Law in quantifying the enthalpy change for a stepped reaction using standard enthalpy change data and bond energy data, for example: carbon reacting with oxygen to form carbon dioxide via carbon monoxide)
Question 11
Calculate the reaction enthalpy for the formation of anhydrous aluminium chloride
from the following data:
Question sourced from ChemTeam
(L2.2:investigate Hess’s Law in quantifying the enthalpy change for a stepped reaction using standard enthalpy change data and bond energy data, for example: carbon reacting with oxygen to form carbon dioxide via carbon monoxide)
Question 12
The following equations illustrate the commercial production of aqueous nitric acid:
Calculate the total enthalpy change required for the production of 2 moles of nitric acid. (2 marks)
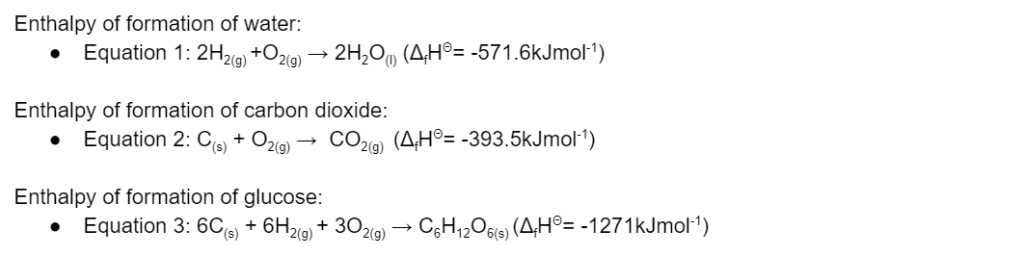
(L2.3: apply Hess’s Law to simple energy cycles and solve problems to quantify enthalpy changes within reactions, including but not limited to heat of combustion, enthalpy changes involved in photosynthesis, enthalpy changes involved in respiration)
Question 13
Using the following information:
Calculate the total enthalpy change of photosynthesis and a respiration reaction. (4 marks)
(L2.3: apply Hess’s Law to simple energy cycles and solve problems to quantify enthalpy changes within reactions, including but not limited to enthalpy changes involved in photosynthesis)
Entropy and Gibbs Free Energy
Question 14
Consider the following statements:
a) Enthalpy is the measure of the internal and flow of energy between objects
b) The system prefers minimum entropy
c) The unit of entropy is measured in JK-1
d) Entropy is an energy
Identify and explain why each statement is either true or false. (4 marks)
(L3.1: analyse the differences between entropy and enthalpy)
Question 15
A student carried out the following experiment:
- Fill a beaker up with 200mL of water
- Increase the temperature of the water such that it is boiling
- Record observations
Justify whether this is an appropriate example of a model illustrating increasing entropy.
(L3.2: use modelling to illustrate entropy changes in reactions)
Question 16
For the following reactions, identify whether it is increasing or decreasing in entropy and explain why. (4 marks)
(L3.3: predict entropy changes from balanced chemical reactions to classify as increasing or decreasing entropy)
Question 17
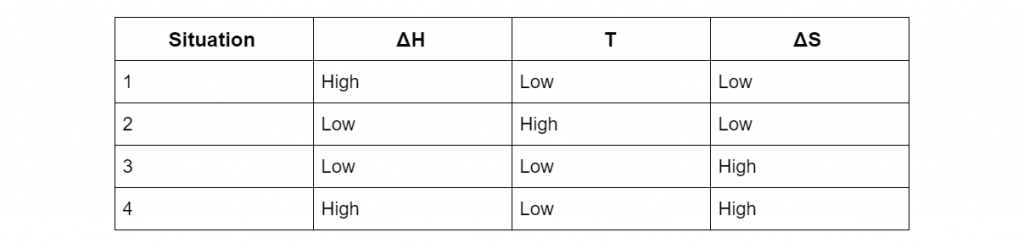
Using the Gibbs free energy formula, deduce whether the reaction would be spontaneous in situation 1,2, 3 and 4 in the table below. (4 marks)
(L3.4: explain reaction spontaneity using terminology, including: Gibbs free energy, enthalpy, entropy)
Question 18
Given the reaction of diamond converting to graphite is 2C(sdiamond)→2C(s(graphite) determine ∆G at 298 K and determine if this reaction is spontaneous or not. What does ∆G say about the rate of this reaction? (3 marks)
∆H°f(C(s)diamond)=1.9kJ/mol
S°(C(s)diamond=2.38J/(molK)
S°(C(s)graphite)=5.74J/(molK)
Question sourced from Chemistry LibreTexts
(L3.5: solve problems using standard references and ??? = ?? ? − ???? (Gibbs free energy formula) to classify reactions as spontaneous or nonspontaneous)
Question 19
Predict and explain whether these reactions would be spontaneous with reference to the Gibbs Free energy formula: (4 marks)
a) Endothermic reaction at high temperatures
b) Endothermic reaction at low temperatures
c) Exothermic reaction at high temperatures
d) Exothermic reaction at low temperatures.
(L3.6: predict the effect of temperature changes on spontaneity)
Question 20
With reference to the Gibbs Free energy, explain why some reactions that go forward are not considered spontaneous reactions. (4 marks)
(Q19-20: L3.6: predict the effect of temperature changes on spontaneity)
And that wraps up our 20 practice questions for Year 11 Chemistry Module 4: Drivers of Reactions – good luck!
- Practice Questions for Module 1: Properties and Structure of Matter
- Module 2: Introduction to Quantitative Chemistry Practice Questions
- 20 Practice Questions for Module 3: Reactive Chemistry
Aiming for a Band 6 in HSC Chemistry? Check out our guide to scoring one here!
Looking for some extra help with Drivers of Reactions?
We have an incredible team of Year 11 Chemistry tutors and mentors who are new HSC syllabus experts!
We can help you master Year 11 Chemistry Module 4: Drivers of Reactions and ace your upcoming HSC Chemistry assessments with personalised lessons conducted one-on-one in your home or at one of our state of the art campuses in Hornsby or the Hills!
We’ve supported over 8,000 students over the last 10 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational HSC Chemistry tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.