Throughout the year, your VCE Chemistry study design is the best checkpoint to track what you’re learning. Or, it can be helpful to check it out before you start the unit to see what you will be covering.
Either way, this article is here to help breakdown VCAA’s learning outcomes for VCE Chemistry.
So what are you waiting for? Let’s get started!
Key Science Skills
Unit 1: How can the diversity of materials be explained?
Unit 2: What makes water such a unique chemical?
Unit 3: How can chemical processes be designed to optimise efficiency?
Unit 4: How are organic compounds categorised, analysed and used?
Key Science Skills
Before delving into the VCE Chemistry content, it is important to take note of the Key Science Skills section. These are skills that relate to planning, conducting and analysing experiments or practical activities.
As you progress throughout VCE Chemistry, it is a good idea to keep these in mind and quiz yourself on them whenever you conduct an experiment in class.
Before you start, ask yourself what is the aim and hypothesis?
As you are collecting your results, question whether they are similar to each other.
Hint: This relates to ‘precision’.
Some of the hardest definitions to nail in VCE science subjects are those relating to scientific investigation, for example, accuracy, repeatability, precision, etc. You can find all of them in this document.
Tip: Create flashcards for these glossary terms!
By doing this, you will slowly become more familiar and comfortable with these terms in the Chemistry study design. This will place you in a great position for the exam, as there is always a question about scientific design testing these skills.
Check out our tips to acing your end of year VCE Chemistry exam!
Unit 1 of the Chemistry Study Design: How can the diversity of materials be explained?
This unit really focuses on introducing you to key concepts of VCE Chemistry which form a foundation for the rest of your studies.
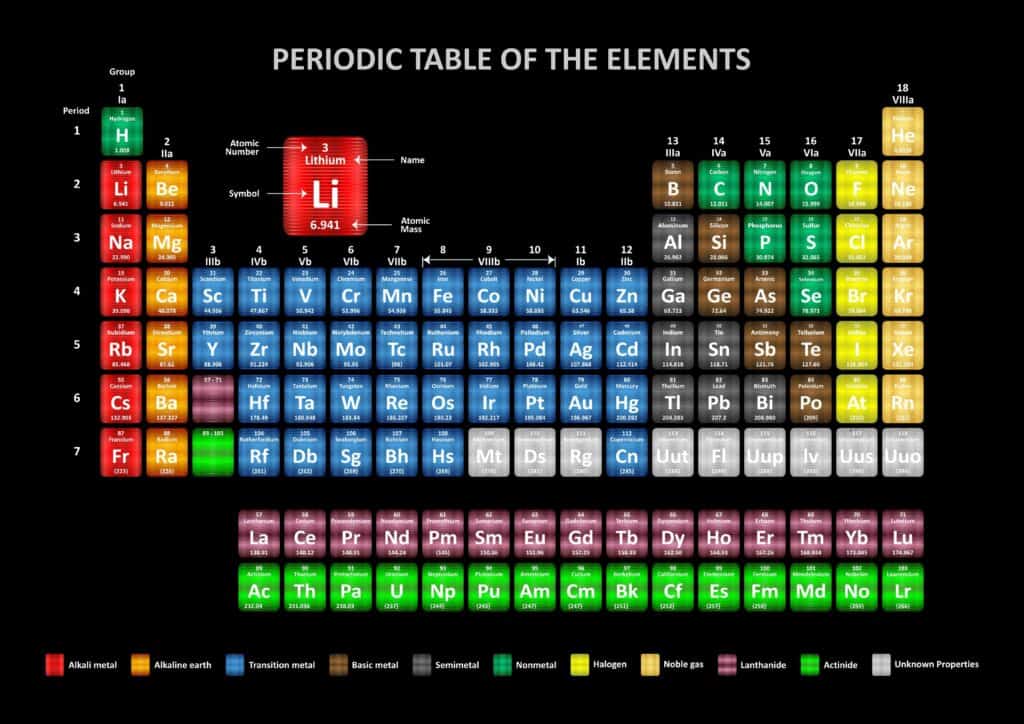
Firstly, the periodic table.
Area of Study 1
To start off VCE Chemistry, you will learn about the nature of chemical elements. You will explore the factors that determine where they sit on the periodic table. The important concepts include:
- Atomic number
- Mass number
- Isotopes
In Area of Study 1, you explore different patterns in the periodic table, based on elements’ properties. A great study idea is to print off a periodic table and colour-code and annotate the patterns, to give you a visual representation of what you are learning. These trends include:
- Atom size/radius
- Electronegativity
- Ionisation energy
- Electron affinity
- Metallic/non-metallic character
- Reactivity
You will learn about how the model of the atom has changed over time. Specifically, you will look into Bohr’s and Schrödinger’s models. In addition, you will learn the electron configuration of the first 36 elements (excluding Copper and Chromium), and learn about electron shells and subshells: s, p, d and f.
You will also look into metals: their properties, their reactivity with other elements, their bonding and structure, and how they can be extracted.
Furthermore, you will learn about the properties, bonding, structure and uses of ionic compounds.
Finally, you will be introduced to very important quantitative concepts: the mole concept, relative atomic mass, percentage abundance, composition by mass and the empirical formula of a compound. These formulas are used all throughout VCE Chemistry, so ensure you spend enough time learning and practising them during this unit.
Area of Study 2
In Area of Study 2, you will be familiarised with different ways of drawing molecules, and understand what features of the molecule each method shows.
- Electron dot formulas
- Structural formulas
- Valence structure
- Ball-and-stick model
- Space-filling model
This will lead on to learning about the shape of molecules, and how this determines its polarity, based on electronegativity, electron-pair repulsion theory and symmetry.
Then you can begin to explain the properties of molecules, including melting and boiling point, conductivity, and softness, as you now understand their structure. To further this, you will learn about intramolecular and intermolecular bonding.
- Ionic bonds
- Covalent bonds
- Dispersion forces
- Dipole-dipole attraction
- Hydrogen bonds
You will discover which type of bond is the strongest and why, and how this can explain the phenomena of ice floating in water.
Not sure how to use the VCE Chemistry Data Booklet effectively? Read our guide before your exams!
This Area of Study also covers the structure, bonding and properties of carbon lattices (diamond and graphite) and carbon nanomaterials (graphene and fullerenes).
Finally, you will be introduced to the basics of organic chemistry. This reappears in Unit 4, so it is important that you put time into grasping the key concepts, which include:
- The hydrocarbon families (and their general formulas): alkanes, alkenes, alkynes, alcohols, carboxylic acids, esters
- Functional groups and their order of significance
- The ways of representing organic compounds: structural formula, semi-structural formula, skeletal formula, Lewis structure
- IUPAC naming system
- Using percentage composition by mass and molecular mass (from Area of Study 1) to determine the empirical formula of organic compounds
- Polymers: their formation (addition polymerisation), linear versus cross-linked polymers, features of linear polymers, advantages and disadvantages of polymer materials
Area of Study 3
This is a research investigation based upon knowledge learnt in either Area of Study 1 or Area of Study 2, and is a great opportunity to practice your key science skills!
There are ten topics to choose from, from which you can develop your own research question or use one of the ones provided by VCAA. This will all be slightly different depending on which school you attend.
Unit 2 of the Chemistry Study Design: What makes water such a unique chemical?
Using your foundational knowledge from Unit 1, you will explore the properties of water, its role as a solvent, and some methods of water analysis.
Area of Study 1
Firstly, you will explore major properties of water:
- Trends in melting and boiling points of Group 16 hydrides
- Specific heat capacity and latent heat (with reference to Hydrogen bonding)
Next, you will look at water as a solvent. A major concept here is precipitation reactions. You need to become familiar with writing full equations for these reactions, with states, using the solubility rules to identify if one of the products is a precipitate.
Then, you are introduced to acids and bases. Here you will learn about:
- The Brønsted-Lowry theory of acids and bases, including polyprotic and amphiprotic species
- The pH scale
- Calculations of strengths of acids and bases, and dilutions of solutions
- Distinction between strong and weak acids and bases, and between concentrated and dilute acids and bases, including examples.
Note: it is super important that you have examples for each of these, and can recognise whether common ionic compounds are strong or weak acids and bases!
- Reactions of acids with metals, carbonates and hydroxides
Finally, you will cover redox reactions in water. This is another concept that you come back to in Unit 3, so it is important that you can understand the basics. You will be introduced to oxidising and reducing agents, conjugate redox pairs, half equations and overall redox equations. This will also be the first time you use the electrochemical series and start to become familiar with formulating balanced redox equations.
Are you scrolling here on the night before your VCE Chemistry exam? Follow our five-step exam prep routine for Chemistry!
Area of Study 2
Firstly, you will explore water sample analysis, what a chemical contaminant is and the processes to avoid contamination.
Then, you will get stuck into measurements:
- Solubility in gram per 100g of water
- The relationship between solubility and temperature
- Solubility curves
- The different ways to measure concentration and how to convert between them: mol/L, g/L, %(m/m), %(m,v), %(v,v), ppm, ppb
Note: These conversions come up in your final exam so try to become familiar with them early on!
Finally, you will be introduced to different methods of analysis. These techniques are used throughout VCE Chemistry in experiments and demonstrations, so, while they can be tricky to understand, really try your best to learn the steps for each method. This will put you in a better position for Units 3 and 4!
- Gravimetric analysis, including the application of mass-mass stoichiometry
- UV-visible spectroscopy/colorimetry, including the use of calibration curves
Note: Calibration curves come up a lot in Units 3 and 4, so it is important to become familiar with them!
- Atomic absorption spectroscopy
- High performance liquid chromatography (HPLC), including learning about the relationship between retention time and polarity
- Volumetric analysis, including the application of volume-volume stoichiometry, and understanding the calculations involved in preparing standard solutions and diluted solutions
- Acid-base titrations to determine an unknown concentration
This Area of Study is notorious for being quite confusing when learning on paper, and is really best understood when you can visualise the analytic processes. It could be a good idea to watch videos of the set-up and steps of each method, or practise in class if you have the opportunity!
Area of Study 3
This is a practical investigation related to water quality, where you plan an experiment and gather data to answer your research question. Another great opportunity to practise and develop your key science skills! Furthermore, it gives you the chance to work through one of the analysis techniques you have just learnt.
Unit 3 of the Chemistry Study Design: How can chemical processes be designed to optimise efficiency?
In this unit, you explore energy resources and manufacturing processes.
Area of Study 1
Firstly, you will learn about fuels. You will learn how to define a fuel, and how to distinguish between different types of fuels. Specifically, fossil fuels and biofuels.
Note: Origin and renewability both need to be mentioned in your definitions of fossil fuels or biofuels to obtain full marks.
Then, you explore different equations and calculations.
- Combustion of fuels: you need to know the balanced equations with states for both complete and incomplete combustion of hydrocarbons, methanol and alcohol
- Thermochemical equations, including enthalpy change
- The universal gas equation, including standard laboratory conditions (SLC)
- How to use specific heat capacity of water to approximate heat energy released during combustion
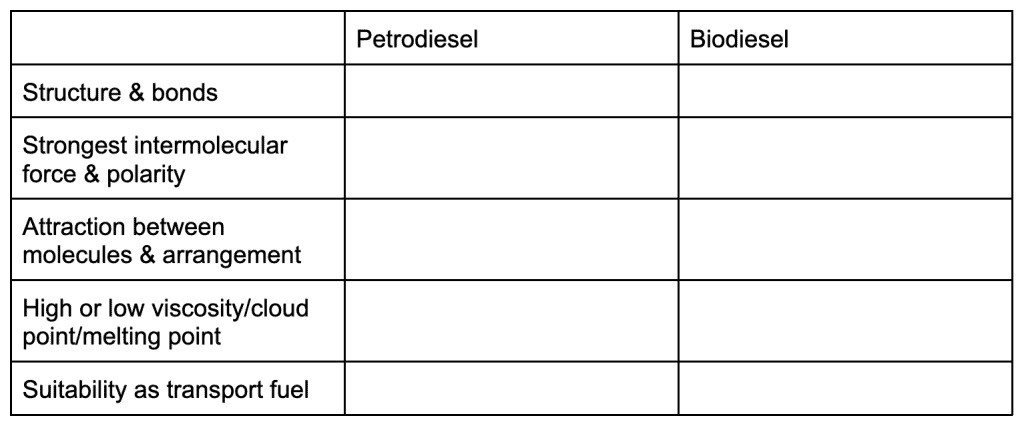
Next, you begin to compare fuels. For this part of the unit, it can be a great idea to draw up tables to help organise your comparisons. You will compare the energy content, renewability and environmental impacts of fossil fuels and biofuels. You will also compare petrodiesel and biodiesel, using your understanding of chemical structures, bonding and polarity to explain their difference in:
- Viscosity
- Hygroscopy
- Cloud point
- Melting and boiling point
Ultimately, you will be asked to discuss which fuel is more suitable for use in a certain environment, and are required to mention the above points. Here is a method of answering these questions, which may help you connect everything:
You will then revisit redox reactions, and build upon your previous knowledge by learning about galvanic and fuel cells. You will need to distinguish between the two in terms of definition, function, design features, energy transformations, energy efficiencies and applications.
Specifically, for galvanic cells:
- Design features: anode, cathode, electrolytes, separation of half-cells, salt bridge, internal and external circuit
- Chemical processes: spontaneous reaction, energy transformation (chemical to electrical), electron and ion (from salt bridge) flows, half-equations and overall equations
- Electrochemical series: you will become familiar with using the electrochemical series to predict products of redox reactions and determining maximum cell voltage
For fuel cells:
- Requirements for electrodes: porous and lined with catalyst
- Comparison between fuel cells’ energy production and producing energy via the combustion of fuels: efficiency, safety, fuel supply, production of greenhouse gases and applications
Area of Study 2
In this area of study, you will explore how to increase the yield and efficiency of chemical processes.
Firstly, in relation to the rate of chemical reactions, you will learn about collision theory. You will learn how to explain how an increase in temperature, volume, pressure, concentration, surface area or addition of a catalyst results in an increase in rate of reaction.
Further, you will learn how to interpret a Maxwell-Boltzmann distribution curve and how to use it to explain how increasing temperature will increase the proportion of successful collisions between particles.
Secondly, you will be introduced to equilibrium equations, and the concepts of equilibrium expressions, equilibrium constants, including how to calculate them. You will then explore Le Chatelier’s Principle in order to explain how the equilibrium system responds to a change by favouring either the forward or reverse reaction, subsequently shifting the position of equilibrium.
In conjunction, you will learn how to interpret and draw concentration-time graphs, and use this knowledge to explain the competing equilibria involved in carbon monoxide poisoning and treatment.
Tip: This example came up on the 2021 VCE Chemistry Exam, so make sure you pay attention to it!
For questions relating to rate and extent of reactions, you will be able to create and memorise a method of answering them. For example, for a question relating to Le Chatelier’s Principle, your answer can follow this method:
- State the effect of the change
- Determine which reaction is then favoured: “According to L.C.P, the system partially opposes the change by favouring the forward/reverse reaction in order to…”
- State which way the position of equilibrium shifts
Finally, you explore non-spontaneous equations (the opposite of redox reactions) by learning about electrolysis and rechargeable (secondary) cells. The key concepts are:
- Features of electrolysis cells: electrode (inert or reactive), electrolyte (molten or aqueous)
- Electroplating
- The use of the electrochemical series in relation to electrolysis and formulating equations
- Comparing an electrolytic cell to a galvanic cell, particularly noting the difference in energy transformation
- Faraday’s Laws
- The requirements for rechargeable cells: products of the discharge reaction remaining in contact with the electrodes, input of energy, products of discharge reaction being in reversible form
- The factors affecting battery life: temperature, side reactions
Unit 4 of the Chemistry Study Design: How are organic compounds categorised, analysed and used?
In your final unit of VCE Chemistry, you will study the way organic structures are represented, named, analysed and processed by the body.
Image sourced from Wikimedia Commons
Area of Study 1
This Area of Study builds upon what you learnt in Unit 1, placing more of an emphasis on the carbon atom.
You will learn about different types of isomers:
- Structural
- Stereoisomers
- Optical isomers and chiral centres
- Cis- and trans-isomers
In addition to what you have learnt about naming organic compounds, you will also learn to explain their differences in boiling point, viscosity and flashpoint based on their structures and bonding. You can approach these questions in a similar manner to questions about petrodiesel and biodiesel (as mentioned above).
Further, you are introduced to organic reaction pathways, which include:
- Oxidation of primary and secondary alcohols
- Substitution reactions of haloalkanes
- Addition reactions of alkenes
- Hydrolysis reactions of esters
- Condensation reactions between a carboxylic acid and an amine
- Esterification reactions between a carboxylic acid and an alcohol
These pathways can become confusing, especially as you also need to learn any catalysts or additional reactants, so it is a great idea to draw a large diagram combining all of these reactions.
Furthermore, you will learn how to calculate atom economy and percentage yield.
Finally, you will explore different ways of analysing organic compounds:
- Mass spectroscopy, including identifying the parent ion peak and determining the molecular mass of the compound
- Infrared (IR) spectroscopy, including using absorption bands to identify the types of bonds present in the compound
- Carbon-13 nuclear magnetic resonance (C NMR) spectroscopy, including analysing chemical shifts to determine the number and type of carbon environments
- Proton nuclear magnetic resonance (H NMR) spectroscopy, including analysing chemical shifts and the area under peaks to determine the number and type of hydrogen environments, and using the n+1 rule to determine peak splitting patterns
- Chromatography, including HPLC and the construction and use of a calibration curve
- Volumetric analysis
You need to know what information each technique allows you to gather, and the chemical principles that allow them to work For example, the spin energy levels involved in NMR spectroscopy.
Area of Study 2
Here, you will study the chemical importance of food.
Firstly, you will learn about the major food molecules:
Proteins
How a dipeptide/polypeptide is formed from the condensation of amino acids, the structures and bonding in primary, secondary, tertiary and quaternary levels of protein, and the distinction between essential and non-essential amino acids
Carbohydrates
How a disaccharide is formed from monosaccharides via glycosidic links, the structure and formation of complex carbohydrates: starch, cellulose, glycogen, and comparisons between glucose, fructose, sucrose and aspartame in regards to their structure and energy content
Fats and oils
Their structural features: ester links, the explanation of different melting points of fats and oils, and different triglycerides due to their fatty acid tails, the distinction between saturated and unsaturated fats, the distinction between essential and non-essential fatty acids, and the structural differences between omega-3 and omega-6 fatty acids
Vitamins
Why they need to obtained via our diet, and the comparison between the structural features of Vitamin C and Vitamin D that determine their solubility in certain substances
Once you understand the different classes of food molecules, you will explore how they are broken down (metabolised/hydrolysed) by the body.
You will explore the structure and function of enzymes as protein catalysts, including their active site, the lock-and-key and induced fit models, enzyme-substrate interaction, and the function of coenzymes. Then, you will look at how changes in pH and temperature affect the enzyme’s performance, in terms of zwitterions, denaturation, and lesser rate of reaction, respectively.
Next, you will study the hydrolysis of starch, and learn why the body cannot hydrolyse cellulose. Further, you will be introduced to the Glycaemic Index (GI) of foods as a ranking system based on the body’s ability to hydrolyse the starch, which is influenced by the proportions of amylose and amylopectin – you will also learn the structural differences between these two molecules.
You will also learn about the hydrolysis of triglycerides (fats and oils) into glycerol and fatty acids, and be introduced to the concept of oxidative rancidity and antioxidants.
Finally, you will study the energy values of the major food molecules, and how to calculate the amount of energy received from a certain food.
Tip: Remember the human body cannot break down cellulose, so we cannot obtain energy from it!
As one final analysis technique, you will study calorimetry, both solution and bomb calirometry, and revise how to calculate the calibration factor and analyse temperature-time graphs.
Area of Study 3
Just like Unit 1 and Unit 2, you will undergo a practical investigation. Depending on your school, you may complete this in Unit 3 or Unit 4.
Major Takeaways from the Chemistry Study Design
Hopefully after reading this guide on the Chemistry study design you can see that the content from each unit flows into the next, and all the foundational concepts you learn early on act as a foundation for the rest of the content that you cover.
Hopefully you can also see that VCE Chemistry is not solely a content-focused subject, and there are many calculations and analysis techniques that are part of the content as well. This means that you need to balance your study time between memorising concepts and definitions, and practising equations and application questions.
Also, don’t overlook the Key Science Skills! There will be a good chunk of marks up for grabs in the exam in relation to these skills, so you really want to feel confident with them.
At the end of the day, the VCE Chemistry course covers a lot of material, so it can be very beneficial to use the Study Design as a checklist as you progress. Not only to keep track of what you have learnt so far, but to also check that you have understood what you’ve learnt and whether you’d feel confident applying it to a VCAA-written question.
Looking for VCE Chemistry past papers? Check out our list here!
Are you looking for some extra help with understanding content from the VCE Chemistry study design?
We have an incredible team of VCE tutors and mentors!
We can help you master the VCE Chemistry study design and ace your upcoming VCE assessments with personalised lessons conducted one-on-one in your home or online!
Get the Chemistry Study Design right, by tutoring with one of our select Box Hill VCE experts! What about considering our VCE Cranbourne tutoring? Get booked in today!
We’ve supported over 8,000 students over the last 11 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational VCE tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Narisha Ford is an avid student who loves all things from studying the immune system, to analysing poetry, and learning how to differentiate logarithms. She graduated in 2021 with a 99+ ATAR and is thrilled to be a part of the AOS Content Writing team to support students through their VCE journey. She hopes to one day work in the international security field, but is still figuring everything in life out!