So it’s the last term of Year 12, we’ve learnt all the basic concepts in chemistry but how do we apply this to real life? HSC Chemistry Module 8: Applying Chemical Ideas translates everything you’ve learnt in the past two years into reality!
In this article, we will be breaking down Module 8 Chemistry: Applying Chemical Ideas through its inquiry questions PLUS give you some pro-tips on how to get a Band 6 for this module!
If you’re looking for some practice questions on Applying Chemical Ideas, make sure you check out our 20 practice questions!
So, what are you waiting for? Let’s jump in!
Overview of Module 8: Applying Chemical Ideas
Inquiry Question 1
Inquiry Question 2
Inquiry Question 3
3 Tips to Get a Band 6 in Applying Chemical Ideas
Overview of Module 8 Chemistry: Applying Chemical Ideas
In Module 8: Applying Chemical Ideas, you’ll learn about how organic and inorganic substances are identified and quantified in our everyday lives.
This is extremely important in ensuring that a certain chemical is within a safe concentration range, ultimately managing the health and environmental risks that come with synthesising products!
If you need to review concepts from HSC Chemistry, make sure you head over to HSC Together which has FREE video resources explaining concepts within each syllabus dot point!
Inquiry Question 1: How are the ions present in the environment identified and measured?
Ever since junior science, we recognise Chemistry by the vibrant colours of test tube solutions.
In Year 12, we will be using these colours to identify inorganic cations and anions through two different methods, the flame test and precipitation test.
Looks like it’s time to revise the precipitation rules from Module 6: Acid/Base Reactions for this topic!
A good tip is to remember the colours of the flame or precipitate that identify a particular cation or anion!
Besides identifying cations and anions, we will also be calculating the concentration of these ions through colorimetry, ultraviolet-visible spectrophotometry and atomic absorption spectroscopy!
Whew, sounds like a handful!
But again, we don’t need to know the complex engineering behind these processes. It’s useful to understand the principle behind each process though.
For example, atomic absorption spectroscopy (AAS) takes advantage of the fact that each ion has their own unique absorbance (extent of absorbing light) in order to quantify its presence in a sample.
Now it’s your turn to ask yourself, which feature of the ion is being exploited in this process? If you’re stuck on answering this we can support you with Chemistry Tutoring across Sydney in person and online.
Inquiry Question 2: How is information about the reactivity and structure of organic compounds obtained?
After we’ve mastered identifying and measuring INORGANIC ions, it’s time to face its more difficult twin, ORGANIC compounds.
Organic compounds such as alkenes, alcohols and carboxylic acids can be identified by their reactivities. It’s important that you understand how these compounds react with others and what the reaction produces.
Here is a table summarising the organic compound, the reaction used to identify them and the product it results in.
| Organic Compounds | Reaction | Product |
|---|---|---|
| Alkenes | Addition Reaction | Alkanes |
| Alcohols | Esterification | Ester |
| Carboxylic acids | Sodium hydrogen carbonate | Salt, water and carbon dioxide |
At times, identifying organic compounds by reactivity is not enough. Reactivity alone does not tell us the structure of the organic compound, which is important because structure determines the function of the compound!
Here comes proton and carbon-13 NMR, mass spectroscopy and infrared spectroscopy to the rescue!
Again, we need to think about the principle behind these processes. While we do not need to know it in detail at HSC level, it is useful to ask yourself, “what part of the organic molecule is being exploited by these technologies?”
For example, mass spectroscopy uses the mass to charge ratio to determine the mass of the organic molecule.
These processes also come with some funky graphs!
It is absolutely essential that you can not only recognise the features of the graphs but also be able to interpret and draw results from them!
Here are some examples of what they look like:
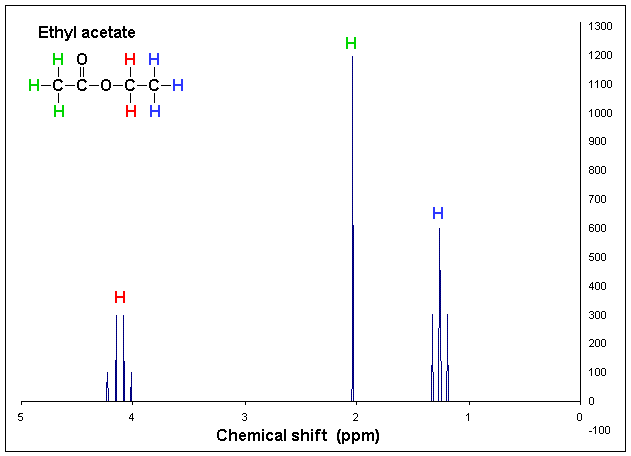
Example of proton NMR graph sourced from Wikipedia.
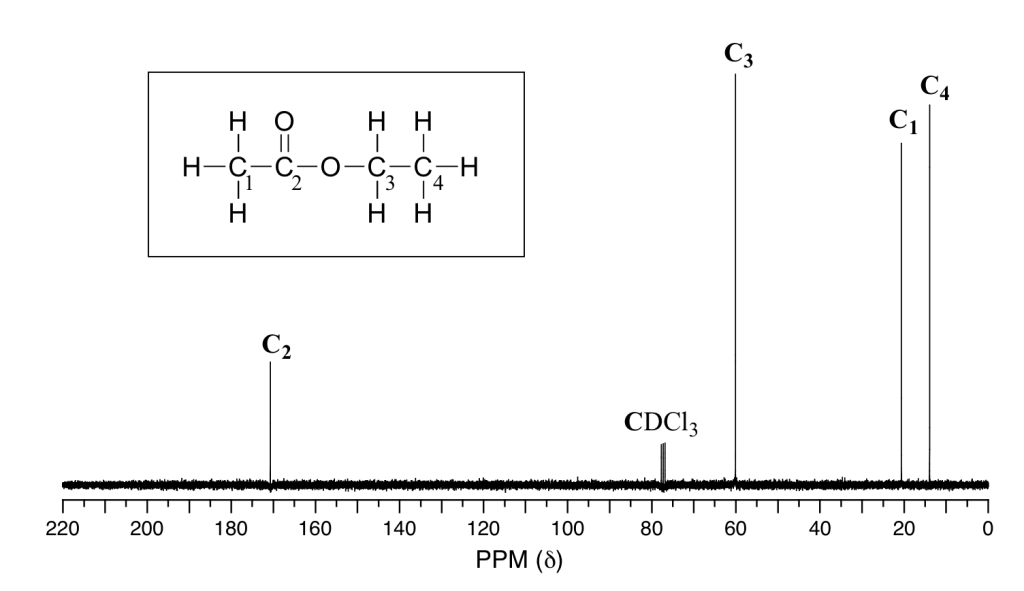
Example of carbon NMR graph sourced from LibreTexts.
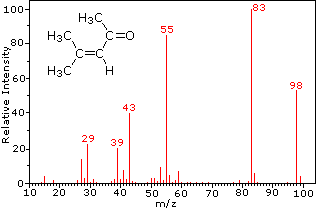
Mass spectroscopy graph sourced from Chemistry MSU.
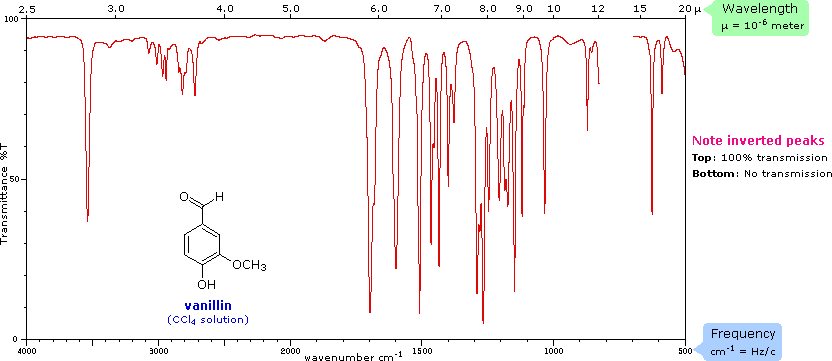
Infrared spectroscopy graph sourced from Chemistry MSU.
Inquiry Question 3: What are the implications for society of chemical synthesis and design?
It’s finally time to take our classroom lessons into the big world!
Here, we tackle the practical complications that come with introducing chemistry into the real world.
We ask ourselves questions such as, do we have enough reagents to meet the demands? What are the reaction conditions required and are they within safe levels?
Most importantly, what are the environmental, social and economical issues that may arise from chemical synthesis?
While it may seem like all these questions are irrelevant to chemistry, it is highly relatable to your building career as a scientist as you start thinking about how your projects may affect people and the world around you.
And this is exactly what the new syllabus wants! The application of theory into real life situations.
Confused? Let our experienced HSC Chemistry tutors help you master every inquiry question and syllabus dot point, prepare for your internal assessments, and be prepared for the HSC Trials and Exams with extensive short answer question practice.
3 Tips to Get a Band 6 in Applying Chemical Ideas
Tip #1: Memorise your reactions!
As you can tell, this module contains a lot of different reactions to identify organic and inorganic substances.
For organic chemistry, a good way to memorise these reactions is to draw a connected flowchart that includes all the types of reactions, with their reactant, products and respective catalysts.
This way you can visually make sense of the overall flow of reactions, while forming a fun summary that is useful for future revision!
For inorganic chemistry, the most you have to memorise is the precipitation rules and the colour of the precipitates. I find that the NAGSAG acronym technique works well in remembering the basic precipitation rules.
Check out a helpful video which presents an easier way to remember the basic precipitation rules!
To memorise effectively, try and test yourself under a time limit!
Tip #2: Identify your mistakes and keep practising!
The key to improving your responses is to check where you have lost your marks! The next step is to ask yourself or your marker why you have lost that mark.
But don’t stop there, identifying your mistakes alone is not enough. It’s always best to try the same question again but with the knowledge of what can be improved.
And practise, practise, practise!
Expose yourself to new questions every week to familiarise yourself with exam style questions and how to write a bomb response.
Luckily for you, the majority of this module remains similar to the old module called Monitoring and Management.
So, there should be plenty of past HSC questions you can access through the NESA website.
Haven’t mastered Module 5 yet? Check out our HSC Equilibrium and Acid Reactions Study Guide to make things 100x easier!
Tip #3: Work with your peers!
Tackling the entire Chemistry module by yourself can be exhausting, especially with organic chemistry being relatively new with limited resources for study.
So don’t feel afraid to work with your peers! Group study can be effective because you can explore different perspectives or explanations that might clarify and solidify your understanding. Plus, your friends might introduce you to new resources that might be difficult to find otherwise.
If you’re still feeling unsure about a topic, question or answer, it’s always best to ask your teacher or your tutor. Especially with the funky spectroscopy graphs, your teacher is always the best to go to as they can be tricky to interpret correctly and justify the results.
Plus, discussing with your teacher or tutor is always handy as they can guide you to write a solid Band 6 response that will blow the socks off your marker!
And that wraps up our guide to acing HSC Module 8 Chemistry: Applying Chemical Ideas – good luck!
Want to ace your other modules too? Check out these Top Study Tips for HSC Chemistry to see results!
Looking for some extra help with Module 8 Chemistry: Applying Chemical Ideas?
We have an incredible team of HSC Chemistry tutors and mentors who are new HSC syllabus experts!
We can help you master the syllabus dot points for Module 8 and ace your upcoming HSC assessments with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby or the Hills!
We’ve supported over 5,000 students over the last 10 years, and on average our students score mark improvements of over 19%!
To find out more and get started with an inspirational HSC Chemistry tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.






