For VCE Chemistry, you receive a data booklet for all your assessments. This is written by VCAA and contains formulas and information to aid you in answering questions. It is very important to become familiar with what your data booklet includes and how to use it – you don’t want to lose any time in the exam!
This article will go through each page of the VCE Chemistry data booklet and discuss how you can effectively use what is provided.
Let’s dive in!
1. Periodic table of the elements
2. Electrochemical series
3. Chemical relationships
4. Physical constants and standard values
5. Unit conversions
6. Metric (including SI prefixes)
7. Acid-base indicators
8. Representations of organic molecules
9. Formulas of some fatty acids
10. Formulas of some biomolecules
11. Heats of combustion fuels
12. Energy content of food groups
13, 14 & 15. Characteristic ranges for infra-red absorption, 13C NMR data and 1H NMR data
16. 2-amino acids
1. Periodic table of the elements
The first page of your VCE Chemistry data booklet contains the periodic table of the elements.
By the time you reach VCE Chemistry, you will be familiar with some of the information that this table offers like the atomic number, element name and symbol, and relative atomic mass of each element.
In VCE, you will learn several trends that you can identify from the periodic table such as electronegativity, atomic radius, and electron shell configuration.
The most common piece of information you will need from the periodic table is the relative atomic mass.
You will often need to use the relative atomic mass in calculations, such as calculating the amount of a substance, so it is important that you can identify elements by both their names and symbols; in your exam the question may only refer to an element by symbol.
2. Electrochemical series
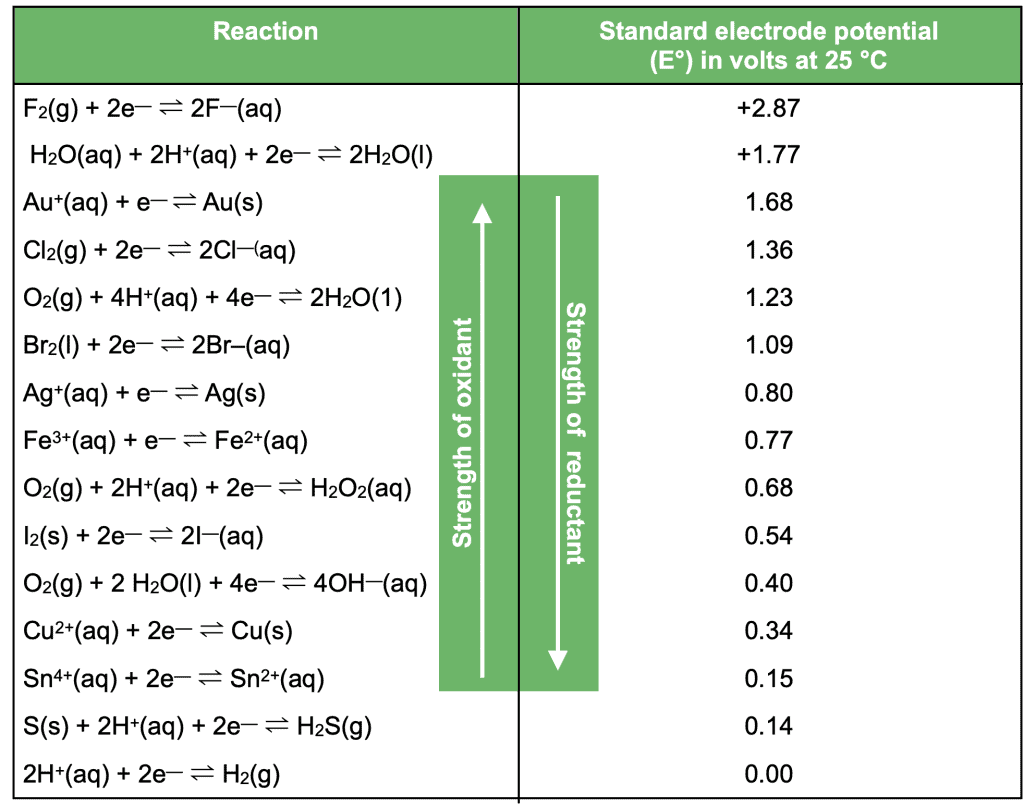
This will be a point of reference for any questions referring to redox or electrolysis reactions. The electrochemical series uses double arrows to show both the reduction and oxidation reaction for each element, as well as its standard electrode potential.
Note: Remember that this standard electrode potential is calculated under Standard Laboratory Conditions
To effectively use your electrochemical series in your VCE Chemistry data booklet, follow these steps to identify the half equations.
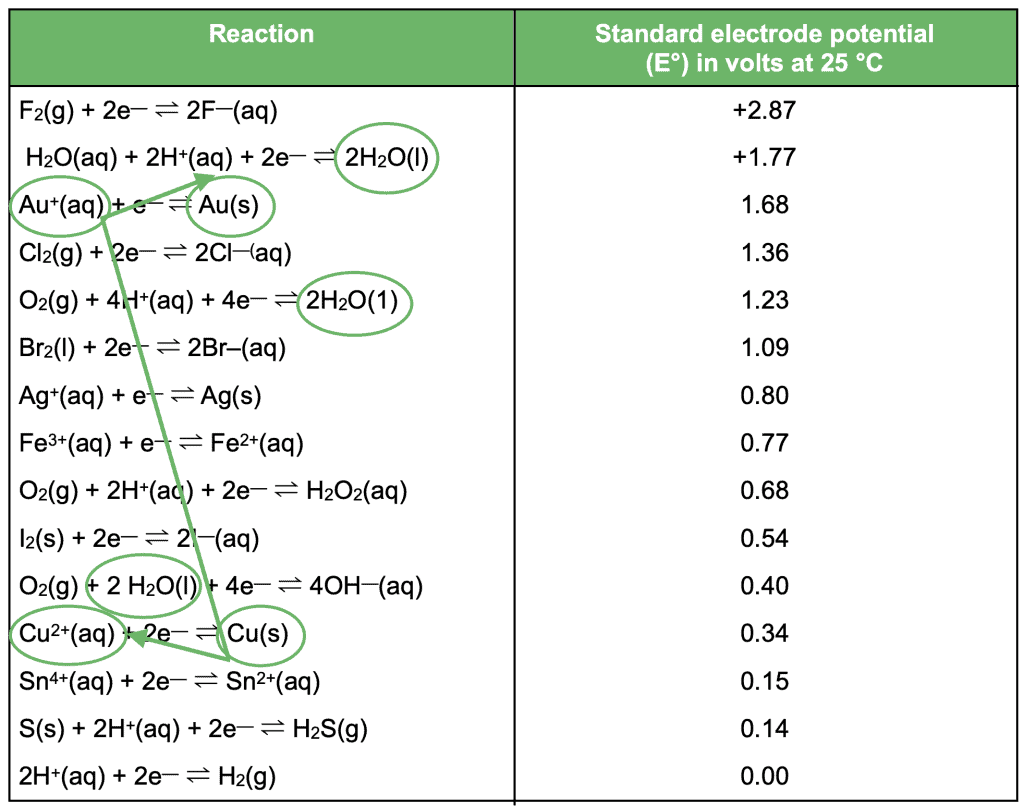
Firstly, circle all reactants that are present in the question – including water in aqueous solutions!
If the question is referring to a redox or spontaneous reaction, draw a backwards Z between the two reactants that gives the biggest gap. For example:
All reactants present are circled in green. Then, find the highest oxidant (on the left) and the lowest reductant (on the right). In other words, the strongest oxidant and the strongest reductant present.
In this example, Au+(aq) is the strongest oxidant and Cu(s) is the strongest reductant.
When we join the two, we can draw a backwards Z showing which direction each reaction occurs in. As a result, we find our two half equations:
Cu(s) → Cu2+(aq) + 2e–
Au+(aq) + e– → Au(s)
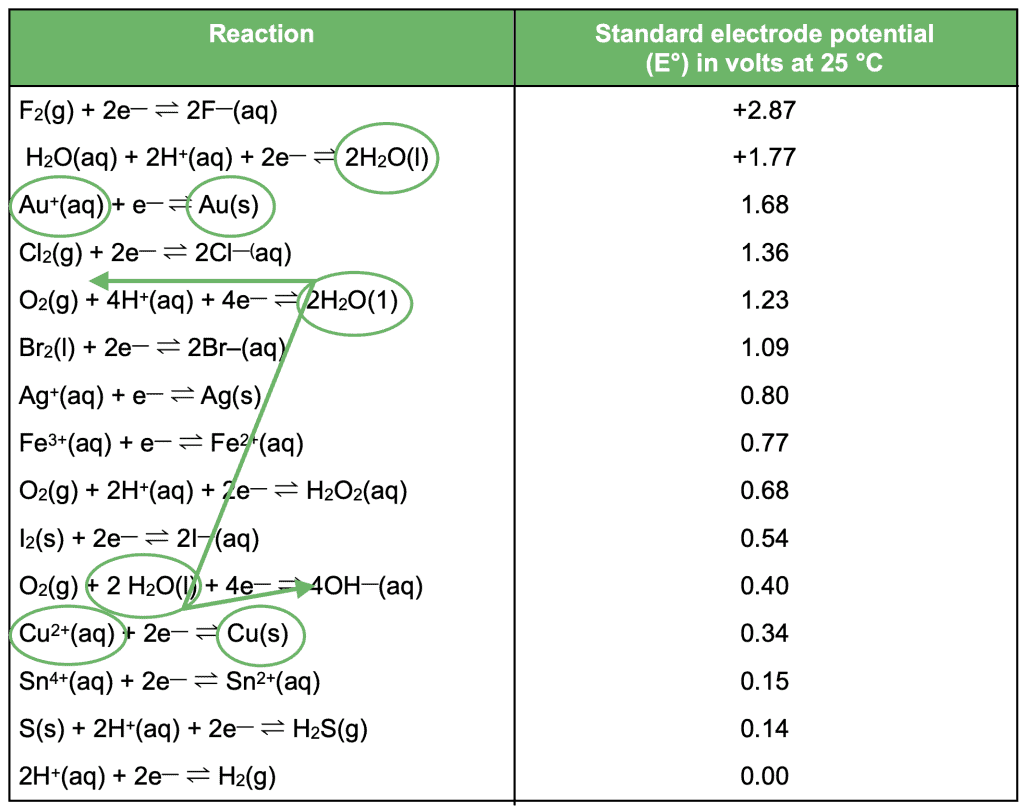
For electrolysis and spontaneous reactions, we go in the opposite direction. We draw a forward Z with the smallest gap. For example:
This time, we choose the oxidant and reductant that produce the smallest gap: H2O
From this Z, we can see the two half equations:
O2(g) + 2H2O(l) + 4e– → 4OH–(aq)
2H2O(l) → O2(g) + 4H+(aq) + 4e–
Once you have your half equations, you need to balance them to ensure they have the same number of electrons.
Cu(s) → Cu2+(aq) + 2e–
(Au+(aq) + e– → Au(s)) x 2 = 2Au+(aq) + 2e– → 2Au(s)
You can then combine them to get the overall equation, cancelling any atoms or electrons they have in common.
2Au+(aq) + Cu(s) → 2Au(s) + Cu2+(aq)
The other piece of information you may need to use from the electrochemical series is the standard electrode potential.
The voltage produced from a reaction is the standard electrode potential of the reductant minus the standard electrode potential of the oxidant. This is the formula:
E0 of cell = E0 (reductant) – E0 (oxidant)
If a question is referring to a reversible or secondary cell and asks you the required energy input, the answer is slightly more than [the E0 of the cell].
Make sure you familiarise yourself with these steps to ensure you can effectively use the electrochemical series in your VCE Chemistry data booklet to gather half equations. It is also important that you know the direction of increasing oxidant strength and increasing reductant strength.
3. Chemical relationships
This section of the VCE Chemistry data booklet gives you some key formulas. While this is very helpful as you don’t have to memorise these formulas, you do need to know what units each of them are in.
Formula Units
n = m/M n: mol, m: grams, M: relative atomic mass
n = V/Vm n: mol, V: litres, Vm: always 24.8L/mol
n = c x V n: mol, c: M (molarity), V: litres
Universal gas equation: p x V = n x R x T p: kPa, V: litres, n: mol, R: always 8.31 J/mol/K, T: Kelvins
Q = I x t Q: C (charge in Coulombs), I: amps, t: seconds
q = m x c x ∆T q: Joules, m: grams, c: specific heat capacity (will be given or if water: 4.18 J/g/K), ∆T: Kelvins
You also need to be comfortable rearranging each of the formulas, depending on what the question is asking you to find.
Often, the best way to ensure you are using the formula correctly is to copy it out from the data booklet and then substitute the information that the question has given you (in the correct units). Then, rearrange to find the unknown.
4. Physical constants and standard values
These are constants that may appear in your calculations and always have the same value. For this section, you really just need to know which formula each of the constants appear in and make sure to substitute them in correctly.
5. Unit conversions
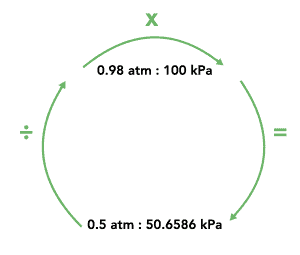
These conversions are especially important when the formula you need to use requires a different unit to the one the question has provided. An effective way to do conversions is shown below:
First, write the ratio given to you. Then write the value you are trying to convert below the number with the same unit. Divide by the number above, and then multiply by the number next to it. This will give you the converted value.
6. Metric (including SI prefixes)
This table helps if you are ever unsure as to what a prefix means in numbers. For example, you may come across nanometres and micrometres in your exam. This table in the VCE Chemistry data booklet can help you convert between units.
7. Acid-base indicators
You will need to use this table to decide which indicator to use in titrations and volumetric analysis. To use this effectively you need to first figure out the range of values which the equivalent point occurs in. This will lead you to a certain indicator from the list.
Next, you may need to determine which direction the colour change occurs. It is important to note that the colour changes listed in your data booklet occur when the solution starts from a lower pH and becomes higher. This isn’t always the case so you need to double check.
Want a comprehensive guide to VCE Chem? Read ‘Everything You Need to Know about VCE Chem’ here!
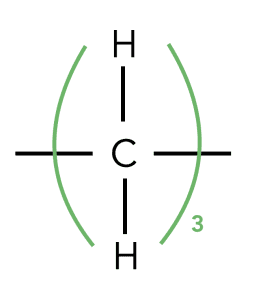
8. Representations of organic molecules
This table is a friendly reminder of the different ways you may be asked to demonstrate an organic molecule in VCE chemistry. If you are ever unsure in the exam, especially with skeletal formula as this can be tricky, consult this page.
9. Formulas of some fatty acids
The main thing to keep in mind with these fatty acids is paying attention to the bonds when drawing them, as there are many repeats in the fatty acid tails. If you draw them in semi-structural form, you can put brackets around a section that is repeated, ensuring that the bonds extend past the bracket. Like so:
10. Formulas of some biomolecules
This section in the VCE Chemistry data booklet is very helpful as it saves you from memorising many molecules, however there are some key molecules not included here which you need to remember, particularly galactose and maltose.
Furthermore, make sure you are familiar with identifying bonds and structures which give each molecule their properties. For example, why Vitamin C is polar while Vitamin D is non-polar, or why amylopectin has a higher GI than amylose.
11. Heats of combustion fuels
You will need to use the values in this table when determining thermochemical equations. Just keep in mind mole ratios; these values are calculated based on one mole of the fuel.
The values are all positive because they represent the amount of heat energy released, however, it is important to remember that when you deal with enthalpy of combustion (∆H) your value could be positive or negative depending on whether the reaction is endothermic or exothermic.
Also, it is important to note that we need to use heat of combustion kJ/g instead of molar heat of combustion when dealing with mixtures, as we cannot determine the molar mass of a mixture. In saying this, section 12 gives you the heat combustion of three common blended fuels.
Also studying VCE Physics? Check out our complete guide here!
12. Energy content of food groups
This table is useful when calculating the energy content of a food based on its components. The formula is quite simple: you multiply the amount of the food group (grams) by its corresponding energy content value in the table.
The one thing to remember is that cellulose cannot be broken down by the human body as we lack the enzyme, so we cannot include it in our calculations. If the question indicates that cellulose is included as a subcategory of carbohydrates, we must take away the amount of cellulose from the total amount of carbohydrates before multiplying.
13, 14 & 15. Characteristic ranges for infra-red absorption, 13C NMR data, and 1H NMR data
The next three sections of the data booklet are vital for determining a molecule from spectra data.
Firstly, you can determine the type of bonds present from analysing an infra-red spectrum. Find the range of wavelength values where the absorption peak(s) occur and consult the data booklet’s table to figure out which type of bond is therefore present in the molecule.
Then, the 13C NMR and 1H NMR data will help you identify the types of carbon and hydrogen environments present. The NMR spectra will show you the number of carbon/hydrogen environments (based on the number of peaks), the ratio of atoms in each environment (based on peak area), and the type of environment present (based on chemical shift) — this is what the data booklet’s table is based off.
Using the chemical shift (ppm away from TSM) of a peak, you can identify the carbon or hydrogen environment.
Note: For the 1H NMR data, the H that is bolded is the hydrogen atom that the chemical shift corresponds to.
For these types of questions, you will need to collect all of these pieces of information and then put it all together to determine the unknown molecule.
Follow our exam prep routine for the night before the VCE Chemistry exam here!
16. 2-amino acids
This table of amino acids contains their full name, symbol and simplified structure.
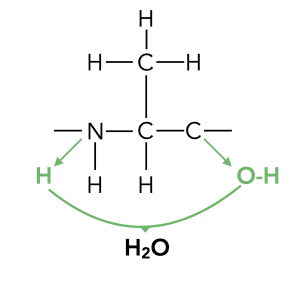
You may be asked to identify the products of hydrolysis of a protein, or conversely, you may be asked to draw a section of a polypeptide chain. Either way, you need to know how to ‘open’ the bonds of an amino acid. Like so:
You will also need to know how to draw a zwitterion. Always check what pH the question is referring to — if it is very low or very high, you will need to check whether you can ionise the side chain.
The best way to use this section of the VCE Chemistry data booklet is to draw the full structure of the amino acid, while keeping the side chain semi-structural. This will make it easier to break or form bonds between amino acids, as you can visualise what is happening.
Do not worry about trying to memorise what each amino acid looks like, however, some common ones to become familiar with are alanine, glycine, glutamine and aspartic acid. Just make sure that you check the side chains very carefully in your assessments, as some amino acids are very similar.
Looking for VCE Chemistry past papers? Check out our list here!
There you have it!
Now that you have some tips and tricks for using your data booklet, make sure to practise these techniques when you complete practice questions.
Becoming familiar with your data booklet early on will save you a lot of time in the exam and ensure that you make the most of what VCAA is giving you!
Follow our steps to acing your end of year VCE Chemistry exam here!
Are you looking for some extra help with understanding content from the VCE Chemistry data booklet?
We have an incredible team of VCE tutors and mentors!
We can help you master the VCE Chemistry study design and ace your upcoming VCE assessments with personalised lessons conducted one-on-one in your home or online!
Don’t get stuck prepping for your VCE Chemistry exam alone! Get a local Box Hill tutor to help you in your preparation! Or book in with one of our Cranbourne VCE mentors!
We’ve supported over 8,000 students over the last 11 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational VCE tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Narisha Ford is an avid student who loves all things from studying the immune system, to analysing poetry, and learning how to differentiate logarithms. She graduated in 2021 with a 99+ ATAR and is thrilled to be a part of the AOS Content Writing team to support students through their VCE journey. She hopes to one day work in the international security field, but is still figuring everything in life out!