
With the new HSC Chemistry Module 7 and its focus on Organic Chemistry, we often see and investigate compounds containing Carbon and Hydrogen atoms, the trademark atoms of organic compounds.
Organic compounds exists all around us and in us too, as we are constantly making, breaking and using organic compounds to do basic things like eating, sleeping and scrolling through Instagram.
In this article, we will be exploring newly introduced topics, a breakdown of Module 7: Organic Chemistry by its inquiry questions with some tips on how to get a Band 6 for this subject!
So let’s get started!
Changes to Module 7: Organic Chemistry
Breakdown of Module 7: Organic Chemistry
How to Get a Band 6 for in Module 7: Organic Chemistry
General Changes to HSC Chemistry Module 7: Organic Chemistry
Previously, organic chemistry was scattered throughout different modules in the old syllabus.
The new HSC syllabus grouped all chemical reactions involving organic compounds into one cohesive module.
Its refined focus on organic compounds illicit a deeper understanding of how structural and chemical compositions can determine the properties of substances. More importantly, how properties of a substance can decide its reactivity with other similar or different compounds.
In Year 11 Module 3: Reactive Chemistry, we learnt about how INORGANIC products are formed by reactions such as synthesis, combustion and acid/base reactions.
In Year 12, we will now be applying this understanding of product formation with ORGANIC molecules.
As discussed in previous articles, the new syllabus has adopted a more depth-over-breadth exploration of concepts in chemistry; prompting understanding and application over route learning.
With Organic Chemistry taking to the stage, students need to understand how chemical structures can determine function and reactivity with other molecules.
Breakdown of Module 7: Organic Chemistry
Before we dive into the inquiry questions, if you need to review concepts from HSC Chemistry, make sure you head over to HSC Together here which has FREE video resources explaining concepts within each syllabus dot point!
Inquiry question #1 : How do we systematically name organic chemical compounds?
“Say my name… say my name.” Most organic compounds are fans of Destiny Child’s hit song, as the require you to name them according to the IUPAC rules.
While naming a structural formula that looks like a tree might be scary, the rule of organic compound nomenclature can be pretty simple.
The first step is to remember the names of compounds with 1 carbon atom, 2 carbon atoms, etc. This is summarized in the table below:
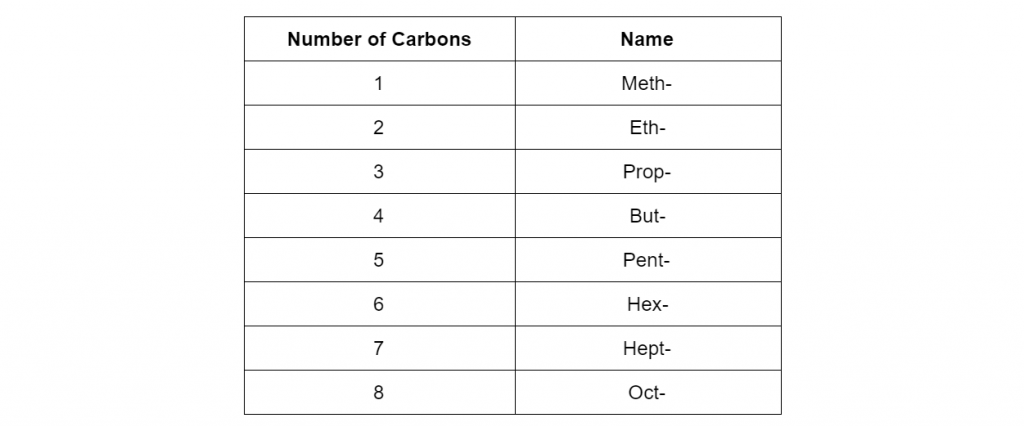
Second step is to recall the structural differences between organic substrate types such as alkanes, alkenes, alkynes, alcohols etc. Apply this to your naming scheme.
Third step is to label groups (bromo, fluoro, methyl) by the nth Carbon atom they are attached to. Remember prefixes! If you have two of the same group, it is called eg. di-fluoro. If you have three of the same groups, it is called eg. tri-fluoro and etc.
Fourth and final step is to ensure the groups are sequenced in alphabetical order in the name.
If your’e struggling, you can find an example of how to name hydrocarbons here, a quick video introduction to the topic here and lastly, a tutorial to naming organic compounds here! We also provide personalised HSC Chemistry Tutoring across Sydney if you are looking for more tailored support.
Inquiry question #2: How can hydrocarbons be classified based on their structure and reactivity?
Organic chemistry compounds such as alkanes, alkenes and alkynes are very similar to real life friendships and relationships in terms of reactivity and bond strength.
While friendships persevere due to a strong single bond, organic molecules also interact less with others when they have a strong bond between the atoms within their structure. Meanwhile, like friendships with two or more people, organic compound with two or three bonds tend to be highly reactive and react with other molecules.
Alkanes (single bond), alkenes (double bond) and alkynes (triple bond) are respectively increasing in reactivity.
This is because alkanes, with a strong single bond is stable without the presence of a reactive double or triple bond. Alkenes on the other hand, have an unstable double bond that is itching to react with another compound. The same can be said about the triple bond of alkynes, who cannot wait to find another friend to react with.
Note: Alkanes with single bonds are known to be SATURATED with hydrogens. Meanwhile, Alkenes with a double bond(s) and Alkyenes with triple bond(s) are known to be UNSATURATED.
Inquiry question #3: What are the products of reactions of hydrocarbons and how do they react?
So what else do organic molecules interact with?
Following from the friendship dynamics of organic molecules, alkanes with a strong single bond remain loyal and is not likely to react with other molecules.
This is partly because alkanes is already SATURATED with Hydrogen atoms!
As alkanes are loyal organic molecules, the only way for it to react with another molecule is to substitute one of its Hydrogen atoms with another chemical substance via substitution reaction.
This chemical substance could be either hydrogen, halogens, hydrogen halides or water.
Alkenes and alkynes can also interact with these chemical substances too. Unlike alkanes however, alkene and alkynes can easily interact with these substances because of their reactive double/triple bonds and UNSATURATED status.
To make alkenes and alkynes saturated, these organic compounds react with other substances via addition reaction.
Haven’t finished your notes for Module 5 yet? We’ve created a full study guide to Equilibrium and Acid Reactions to make things 100x easier!
Inquiry question #4: How can alcohols be produced and what are their properties?
OH, OH, OH it’s alcohol!
Alcohol is a similar to alkanes, but instead of a normal boring hydrocarbon, it has an hydroxyl (OH) group.
This hydroxyl (OH) group induces intermolecular forces such as hydrogen bonds, dipole-dipole forces and dispersion forces.
This OH group is handy in performing oxidation reactions, dehydration and even preventing incomplete combustions!
Because of alcohol’s many uses, it has a high demand! Luckily, alcohol can be produced via substitution reactions with halogenated compounds or fermentation.
Inquiry question #5: What are the properties of organic acids and bases?
Now, before we get too sour about the revisit to carboxylic acids and amino bases such as amines and amides, this topic also includes new groups such as alcohols, aldehydes and ketones.
Structural formulas can be referenced below!
Besides normal acids and bases interactions, we will take on a clean approach to new reactions such as esterification, the formation of perfumes and saponification, the production of soaps and detergents. How exciting!
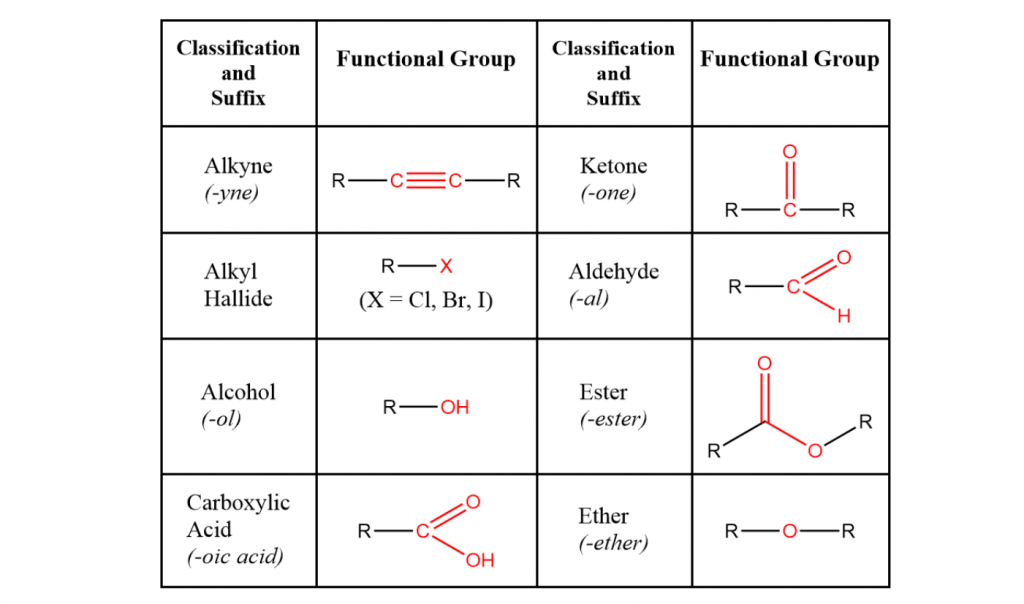
Sourced from Chemistry Score
Inquiry question #6: What are the properties and uses of polymers?
Imagine you have a string. You place one bead into the string, followed by another and another and another. Finally, you stop and tie a knot to the string and you have a bracelet!
That is what polymerization is. The beads represent monomers, individual organic compounds that add on and on. The process of adding on monomers (beads in the metaphor) is called polymerization. Once that has ended, polymerization has terminated and you have a bracelet, or in chemistry terms – a polymer.
Polymers are in what you use in everyday life! From the polystyrene that keeps your food warm to the polyesters in your shirts, polymers make up every bit of our living.
That is why it is important to see how its structure, composition and interaction with others make up your clothes, containers and whatever else you can think of!
How to Get a Band 6 for in HSC Chemistry Module 7: Organic Chemistry
Tip #1: Memorise your nomenclature and structures!
To master the Band 6 content, it is always best to master the basics first.
To memorise effectively, try and test yourself!
Attempting to write names or draw structures for many different types of organic molecules will broaden your scope. Soon enough, any molecule in your way will be a breeze for you.
Feel free to take note of any organic compounds that you struggle to name or draw. Discussing them with your teacher is a great way to confirm which nomenclature or structure will earn you the most marks in the exam!
Tip #2: Collaborate with your peers and teachers
Because some of the concepts are new in HSC Chemistry Module 7, it may be difficult to access relevant practice questions and their corresponding answers.
Collaborating with friends is a great way to share your resources and understanding with one another. Perhaps their way of explaining may open your eyes to a whole new way of seeing and remembering the concepts you previously struggled with. Perhaps they can present to you resources that you couldn’t find otherwise.
Also don’t feel afraid to approach your teachers whenever you are unsure about a question, topic or answer. Discussions with teachers are always handy as they can give you clues about what your marker is expecting from a Band 6 student.
But doing one or two practice questions is not enough. In order to master the topics you have struggled with, it is always best to practice, practice and practice.
The 2019 updated version of Excel HSC Past Papers workbook is one of the best resources that lists all relevant past HSC questions by module.
So what’s stopping you? Let’s start practising!
Tip #3: Apply theory in your pracs
As you are performing your experiments, try to account for what is being observed.
This means putting the concepts you have learnt in class into action!
A good way to apply your knowledge is to prepare for practicals by re-reading any relevant chemical concepts that forms the basis of the experiment.
That way, you can actively apply what you have read earlier that week to what you see during your practical lesson!
When revising for Module 7: Organic Chemistry, always ask yourself these questions:
- What are the structural differences between these organic molecules?
- How do these structural components contribute to its interaction and reactivity with other molecules?
By asking yourself these questions every time you perform a new reaction in class, you’re essentially applying your understanding of chemistry to many different organic chemical reactions!
Want to ace your other modules too? Start using these Top 5 Study Techniques for HSC Chemistry to see results!
And that wraps up our guide to HSC Chemistry Module 7: Organic Chemistry! Good luck!
Looking for some extra help with HSC Chemistry?
We pride ourselves on our inspirational HSC Chemistry coaches and mentors!
We offer tutoring and mentoring for Years K-12 in a variety of subjects, with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby!
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on Facebook!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.


