
What’s remained static and what’s changed dynamically? That’s the question on everyone’s mind when it comes to the new HSC Chemistry Module: Equilibrium and Acid reactions.
As Equilibrium now plays as a big star in the new Year 12 module, we are not the only ones going back and forth.
That’s right- the current HSC Chemistry Module 5 now centralises itself on Equilibrium, a chemical reaction where the rate of forward reaction (reactants to product) is equal to the rate of reverse reaction (products to reactants).
In this article, we will be exploring newly introduced content, a breakdown of the new Module 5: Equilibrium and Acid Reactions by its inquiry questions and some tips on how to get a Band 6 for this subject!
So, let’s dig in!
Overview of Module 5: Equilibrium and Acid reactions
How Do I Get a Band 6 for this Module?
Overview of Module 5: Equilibrium and Acid reactions
Before we dive into the inquiry questions, if you need to review concepts from HSC Chemistry, make sure you head over to HSC Together here which has FREE video resources explaining concepts within each syllabus dot point!
Inquiry Question #1: What happens when chemical reactions do not go through completion?
There are some chemical reactions that can procrastinate as much as we do.
In Year 11, we familiarise ourselves with the famous hardworker Static Equilibrium that goes through completion, exhausting all its reactants to make its products.
Year 12 brings forth Dynamic Equilibrium whose work ethics goes backwards and forwards, causing leftover reactants and products as the equilibrium reaction does not go through completion.
Inquiry Question #2: What factors affect equilibrium and how?
Like how the pressure, heat and volume of schoolwork affect us students, these factors can also affect how an equilibrium reaction would act.
While students may work harder or less depending on circumstances, an equilibrium reaction will also react to changes in pressure, heat and concentration/volume.
It reacts by either turning backwards or accelerating forwards to cancel out the stressful change in its system.
Check out our Top 5 Study Tips for HSC Chemistry to make your life 100x easier and score that Band 6!
Inquiry Question #3: How can the position of equilibrium be described and what does the equilibrium constant represent?
“To the left, to the left”. Extending from Queen Bey’s iconic line, equilibriums can be positioned either to the right (forward reaction) or to the left (reverse reaction).
But how do we know which direction it goes?
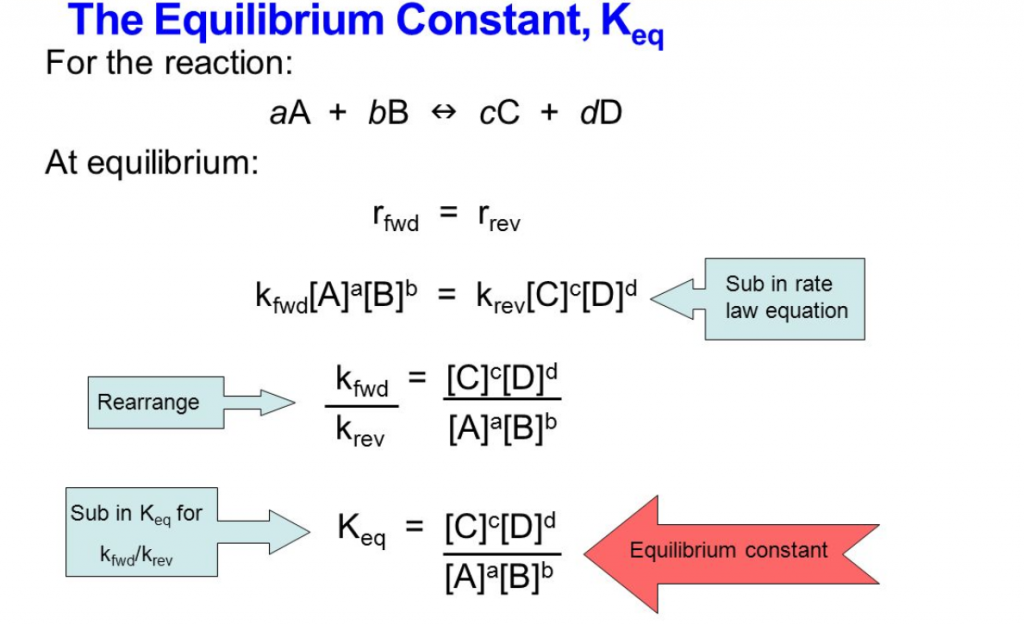
Coming to our rescue is our soon to be best friend, the equilibrium constant, Keq.
Keq determines the position of the equilibrium by calculating the ratio of concentration of products over the concentration of reactants.
Sourced from Slide Player: Equilibrium SCH4U organic photochromic molecules respond to the UV light by Oswin Banks
Simply saying, the bigger the Keq, the more likely the reaction will proceed forwards. Similarly, the smaller the Keq, the more likely the reaction will move backwards.
Inquiry Question #4: How does solubility relate to chemical equilibrium?
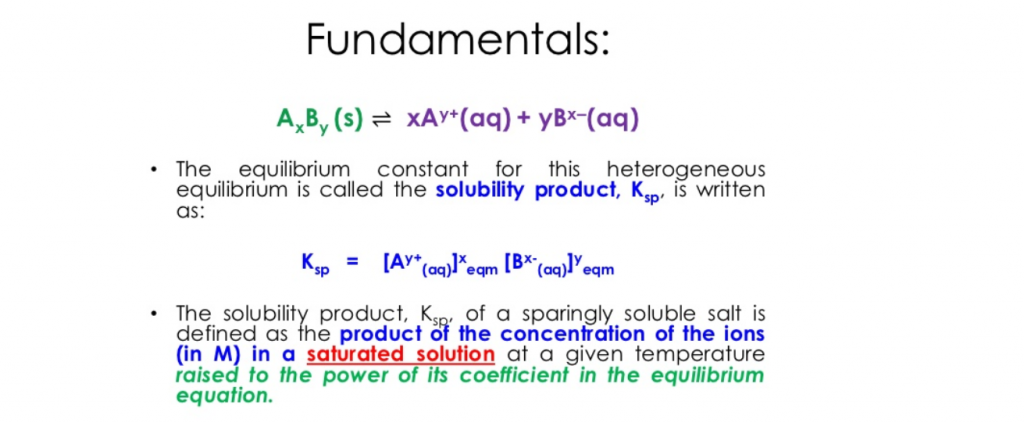
Now we’ve met Keq, let’s get to know its brother Ksp. But who is Ksp?
Ksp is the equilibrium constant for the solubility of a substance. In other words, the level at which a solute dissolves into its solvent.
But how is solubility related to equilibrium?
Just like its sibling Keq, a bigger Ksp constant will indicate a higher likelihood of a forward equilibrium reaction and therefore, a higher capacity to dissolve.
A smaller Ksp will indicate a lower likelihood of a forward equilibrium reaction and lower likelihood to dissolve.
Unlike Keq however, calculating Ksp would require only the multiplication of the concentration of products, as seen in the formula below:
Sourced from Slide Player: CM4106 Review of Lesson 4 by Yong Yao Tan
Don’t wait until it’s too late to master this module! Let our experienced HSC Chemistry Tutors support your studies at our Campuses in Hornsby or Castle Hill, at your home or online.
How Do I Get a Band 6 for this Module?
Tip #1: Map out equilibrium
Just like the module, your mind map will encompass all things to do with our central focus, Equilibrium.
Grab the largest piece of paper from your nearest Officeworks or newsagency, and start mapping out all things you know about Equilibrium!
Design your mind map so that each branch explores its respective inquiry questions.
This way you can learn how one part of the module relates to another, forming a big picture so it can be used as an umbrella summary for future revision.
Feel free to use sticky notes as well to add solutions for harder questions (calculations, trickier concept questions, etc) to the mind map so you can easily look back at what you need to master.
Tip #2: Identify holes in your understanding
The new NESA website has updated the HSC past papers from 2018 to the new syllabus.
It’s always best to do as many as these papers as possible to get a feel of the exam question styles and the level of thinking and understanding required to ace band 6 questions.
Not enough time to complete papers? No worries.
The workbook, Checkpoint Chemistry is an amazing resource that delivers all the tricky questions in past HSC papers.
It includes both multiple choice and long response, along with marking guidelines so you can familiarise yourself with what your marker is expecting from a Band 6 student.
But identifying what you don’t understand is not enough! You need to master the topics you are struggling with and the best way to do that is to practice, practice and practice!
So what are you waiting for? Get grinding!
Tip #3: Apply theory in your pracs
As you are writing your practical report, ensure you are explaining what you see.
This means applying the concepts you’ve learnt in class to what you observe in your pracs.
If you have any trouble remembering what has happened in class, it is good to revise your practical experiments at home online.
While revising each practical, ask yourself these questions:
- What are the molecular interactions that have caused the equilibrium position to shift?
- How has outside factors affected the molecular interactions within the reaction?
By asking yourself this question every time you see a change in the reaction, you’re essentially mastering your understanding of chemistry on a molecular level!
And that wraps up our guide to HSC Chemistry Module 5: Equilibrium and Acid Reactions!
Looking for some extra help with HSC Chemistry?
We pride ourselves on our inspirational HSC Chemistry coaches and mentors!
We offer tutoring and mentoring for Years K-12 in a variety of subjects, with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby!
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on Facebook!


