With the new syllabus promoting application of theory over rote-learning, you might be assigned to write an investigation or research report for HSC Chemistry.
Though it might sound like heaps of work, it is a useful head start for your future studies and careers in STEM!
Learning how to plan experiments and structure your report are key skills in the field of STEM, as experimental reports are required very often for systematic reviews, future improvements and many other important uses.
In this article, we’ll be equipping you with a step-by-step guide on how to write a Research Report designed for HSC Chemistry – let’s go!
What is a Research Report?
How to Write a HSC Chemistry Research Report
Step 1: Title
Step 2: Abstract
Step 3: Introduction
Step 4: Methods
Step 5: Results
Step 6: Discussion
Step 7: References
What is a Research Report?
Simply put, a report is a written article that summarises your research and/or experiments.
In the STEM community, reports are useful in communicating new concepts, methodology or even suggestions for improving future experiments.
For Chemistry, most reports are used to convey an interpretation of the experimental observations with reference to existing theories. While quantitative data (measurements and calculations) can be important, qualitative results (physical descriptions) are also useful in figuring out what is going on behind the scenes.
A research report is perhaps the most succinct method that encapsulates all aspects of your research.
Here are some points to consider from NESA’s Chemistry Stage 6 Assessment and Reporting outline:
As NESA states, your class may be allocated to write a research report as your depth study.
Ultimately, your research report needs to include your hypothesis, methods, results and discussion. Let’s flesh this out a little more!
How to Write a HSC Chemistry Research Report
Luckily, most scientific disciplines share similar structures and formatting with slight differences at times.
To help you, we’ve composed a simplified guide on writing a HSC Chemistry research report in just 7 steps!
Step 1: Title
The title of your report should incorporate your main variables and subject matter.
This means explicitly including your dependent variable, independent variable and the reagent(s) you will be testing.
Example:
The time it takes for 1cm2 of rust to form on the element iron versus the alloy bronze as a measure of oxidation rate.
Note: Ensure that any scientific notations or symbols used are presented correctly as markers can be nit-picky about this.
Step 2: Abstract
The abstract is a summary of your experiment, specifically your purpose, your results and how your results were gathered.
Abstracts should be:
- A paragraph long (< 200 words)
- Include title, background, aim, methods, results and conclusion.
- Clear and succinct.
You can find examples of Chemistry abstracts here!
Step 3: Introduction
There are so many things to include in your introduction.
What is/are your research focus/es? What are the background concepts that underpin your research question? What is/are your hypothesis/es?
Here is a checklist of what your introduction should include:
- Your research question
- Relevant knowledge and background information about the subject of research question
- Your hypothesis based on current literature
- Your experimental approach
- The beneficial contribution of your research to scientific advancement
Note: Your introduction must NOT be more than 4 paragraphs long.
Example of hypothesis:
Drawing from the knowledge that alloys generally have a lower oxidation rate than elemental metals due to alloy’s more diverse properties, it was hypothesized that a bronze alloy will require a longer time to oxidize into rust as opposed to the element iron.
Step 4: Methods
Reaction Equation Section
For experiments that require quantitative analysis, it is usually accompanied by relevant equations.
These equations do not have to be solely mathematical, as they can include chemical formulas too. It can also include the structures and names of products and reactants, as well as the conditions of the experiment (temperature, solvent, atmospheric pressure, etc).
Struggling with Module 5 for HSC Chemistry? We’ve made a study guide on Equilibrium and Acid Reactions for you to use!
Experimental Section
Here in the methods section, you will need to detail the methods you have used to test your hypothesis.
Your protocol should be clear, well-structured and in chronological order.
It is very important that your experiment still remains DETAILED such that scientists can replicate your procedure in future experiments!
If your procedure is complex, consider drawing up a flowchart or illustration to visualise your methods.
Note: Consider the conditions of your experiment (temperature, humidity, etc).
Example:
Bronze alloy and iron metal samples were cut into 5cm x 5cm squares with a thickness of 2mm. Eight samples were prepared. A pair of bronze alloy and iron metal squares were placed in each of the three humidity controlled boxes with varying humidities (25%, 50%, 75%) at room temperature (25°C) to regulate their exposure to water.
Step 5: Results
Here is where you describe your results. You may consider the use of tables and graphs to convey your results but it does depend on whether your investigation produces quantitative or qualitative outcomes.
DO NOT interpret your results in this section! The interpretation of results belong in the discussion section.
Example:
The mean rate of rust production on the bronze sample was 0.3mm per day while the mean rate of rust production on the iron sample was 5mm per day in the 50% humidity environment (Table 1). The mean rate of the rust production of iron sample in 50% humidity environment was 2mm more than the mean rate of rust production of iron in the 25% humidity environment.
Step 6: Discussion
In this section, draw out any significant results and try to account for them.
To really present an in-depth discussion, check if it answers the following questions:
- What are the significant results?
- What do the results mean? What do they represent?
- Do the results accept or reject the hypothesis?
- Do the results agree or disagree with existing scientific literature?
- What are the limitations and improvements on accuracy, reliability and validity that can be made?
- How can this research contribute to future experiments and scientific knowledge?
- Is there a concluding paragraph summarising all of the above?
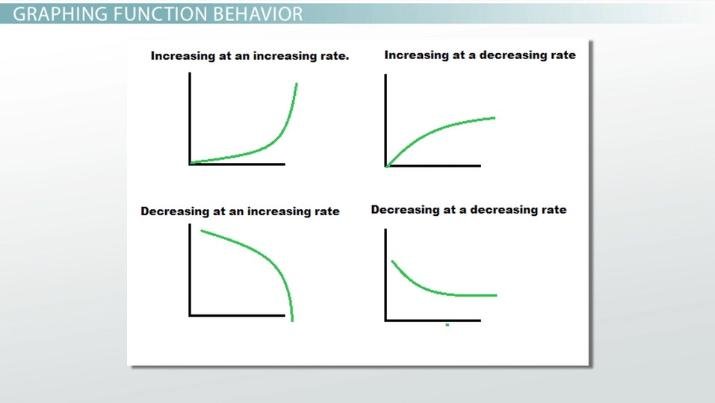
Note: Be sure to describe your significant results in as much detail as possible. For example, when describing trends, describe whether it increases at an increasing rate or decreasing rate. If confused, here is a diagram to show how your graph might look like and how to describe the trends at a higher detail:
Image sourced from Study.com
Example:
The results show that both brass and iron samples rusted faster at an exponential rate in an environment of higher humidification. This could be due to a higher amount of water being available in a highly humid environment, allowing more of the metal molecules to oxidise in the presence of more water. This finding supports our original hypothesis that a higher humidified environment will accelerate oxidation reaction, further substantiating Evan’s (2013) investigation of the mechanism of rusting under varying environmental conditions…
Step 7: References
Here, ensure that all citations of literature you have mentioned or used are listed. Your school might have a different referencing style from other schools, so don’t forget to ask your teacher or check your marking criteria!
Example:
Image sourced from Library Guides
NOTE: This reference list uses the Harvard referencing style.
And that wraps up our 7 step guide to writing an HSC Chemistry research report – good luck!
Looking for some extra help with your HSC Chemistry research report?
We have an incredible team of HSC Chemistry tutors and mentors who are new HSC syllabus experts!
We can help you with your HSC Chemistry research report and ace your upcoming HSC assessments with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby!
We’ve supported over 5,000 students over the last 10 years, and on average our students score mark improvements of over 19%!
To find out more and get started with an inspirational HSC English tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.