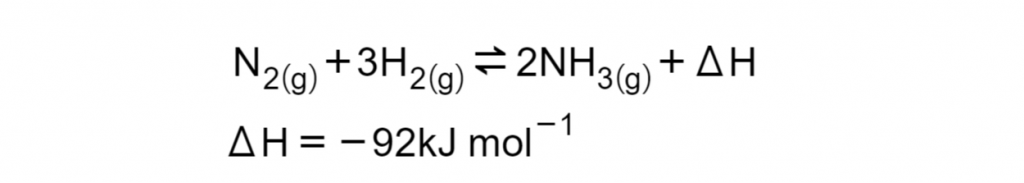Searching for some practice questions on the new HSC Chemistry Module 8: Applying Chemical Ideas?
We’re here to help!
While the questions on inorganic substance analysis are easy to find as they are recycled from the old syllabus, it can be difficult to look for questions on organic substance analysis as they are introduced quite recently.
To help you, we’ve gathered 20 HSC Chemistry practice questions that cover each of the 7 dot points in HSC Chemistry Module 8: Applying Chemical Ideas to help you master your exam responses!
But wait! Have you mastered Module 5 yet? No? Bookmark our study notes for Equilibrium and Acid Reactions first!
Let’s get started on those Module 8 practice questions now!
Analysis of Inorganic Substances
Analysis of Organic Substances
Chemical Synthesis and Design
Analysis of Inorganic Substances
Question 1
Justify the need for monitoring environments such as water bodies (3 marks).
(L1.1: Analyse the need for monitoring the environment)
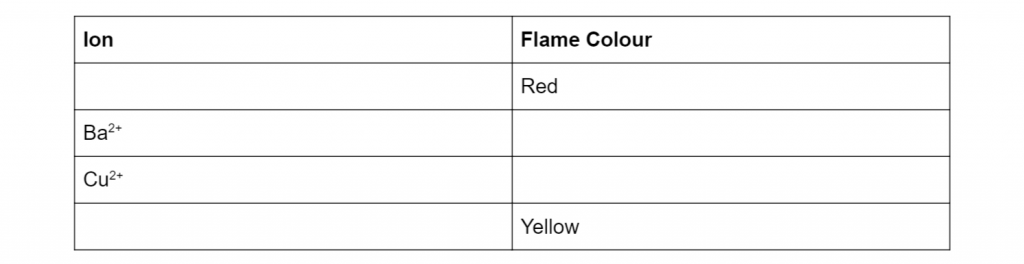
Question 2
Complete the table below with the correct ion and its flame colour (4 marks)
(L1.2: Conduct qualitative investigations using flame tests, precipitation and complexation reactions as appropriate to test for the presence of aqueous solution in the following ions and anions)
Question 3
Can a flame test be performed on Pb2+? Why or why not? (2 marks)
(L1.2: Conduct qualitative investigations using flame tests, precipitation and complexation reactions as appropriate to test for the presence of aqueous solution in the following ions and anions)
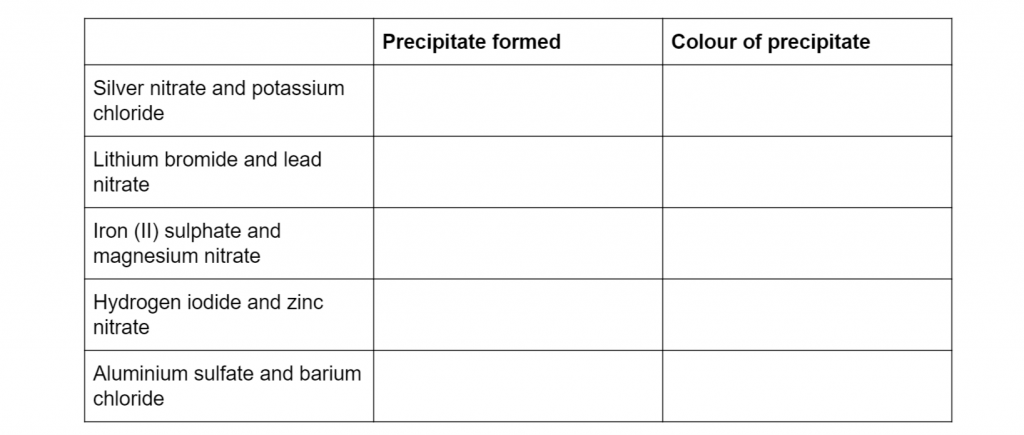
Question 4
Complete the table below, indicating the precipitate formed and its respective colour (10 marks)
(L1.2: Conduct qualitative investigations using flame tests, precipitation and complexation reactions as appropriate to test for the presence of aqueous solution in the following ions and anions)
Question 5
Write out the balanced chemical equations for the reactions above. (5 marks)
(L1.2: Conduct qualitative investigations using flame tests, precipitation and complexation reactions as appropriate to test for the presence of aqueous solution in the following ions and anions)
Question 6
During a gravimetric analysis investigation, you found that the mass of a sample containing KCl weighed 0.628g. This solution was treated with AgNO3 and the subsequent AgCl precipitate weighed 1.345g. Calculate the percentage of K that was in the original sample (4 marks)
(L1.3: Conduct investigations and/or process data involving: Gravimetric analysis, Precipitation titration)
Question 7
A mixture containing KCl and NaBR only is analyzed using the Mohr method. A 0.3172g sample is dissolved in 50mL of water and titrated to Ag2CrO4 end point, requiring 35.85mL of 0.1120 M AgNO3. A blank titration requires 0.71mL of titrant to reach the same endpoint. Report the %w/w KCl in the sample. (5 marks)
Question sourced from LibreTexts
(L1.3: Conduct investigations and/or process data involving: Gravimetric analysis, Precipitation titration)
Question 8
Describe the steps used to determine the concentration of a solution using colorimetry (5 marks).
(L1.4: Conduct investigations and/or process data to determine the concentration of coloured species and/or metal ions in aqueous solution, including but not limited to, the use of: Colourimetry, UV spectrophotometry, Atomic absorption spectroscopy)
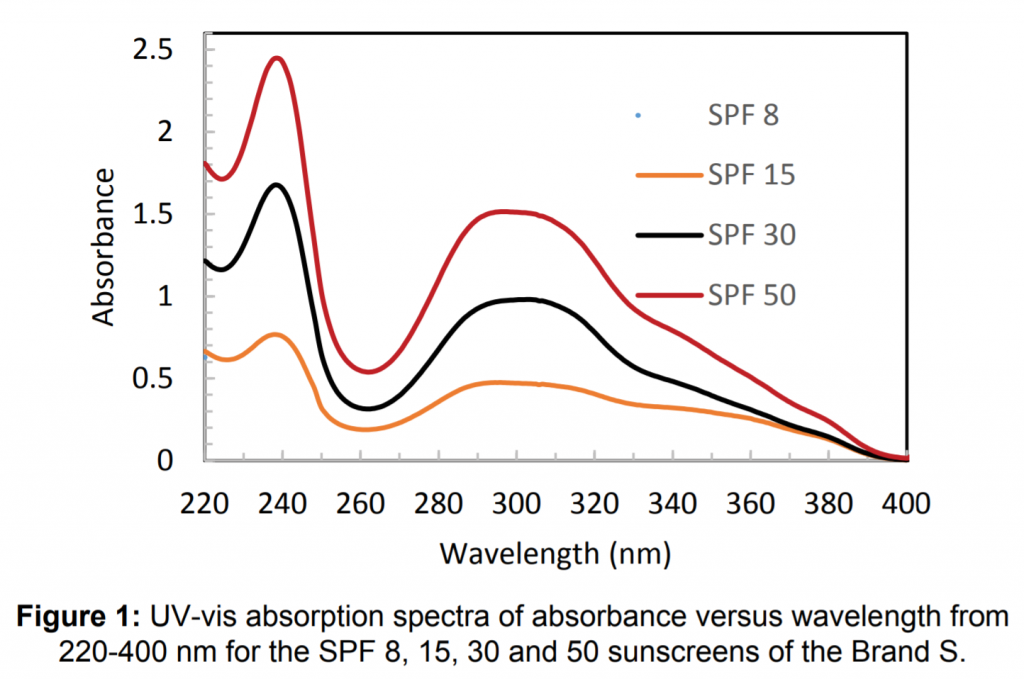
Question 9
The following graph has two peaks, labelled ‘a’ and ‘b’. Answer the following questions using the graph below. (3 marks)
Graph sourced from Chou et. al. (2017)
a) What does the first peak represent?
b) What does the second peak represent?
c) How would you use this graph to quantify the sun protection factor (SPF) of a new sunscreen brand?
(L1.4: Conduct investigations and/or process data to determine the concentration of coloured species and/or metal ions in aqueous solution, including but not limited to, the use of: Colourimetry, UV spectrophotometry, Atomic absorption spectroscopy)
Question 10
Draw a diagram depicting how atomic absorption spectroscopy works. Explain the principle behind this procedure. (4 marks)
(L1.4: Conduct investigations and/or process data to determine the concentration of coloured species and/or metal ions in aqueous solution, including but not limited to, the use of: Colourimetry, UV spectrophotometry, Atomic absorption spectroscopy)
Analysis of Organic Substances
Question 11
Explain how you used bromine water to test for the presence of a carbon-carbon single or double bond. Include relevant structural equation(s). (3 marks)
(L2.1: Conduct qualitative investigations to test for the presence of organic molecules of the following functional groups: Carbon-carbon double bonds, Hydroxyl groups, Carboxylic acids)
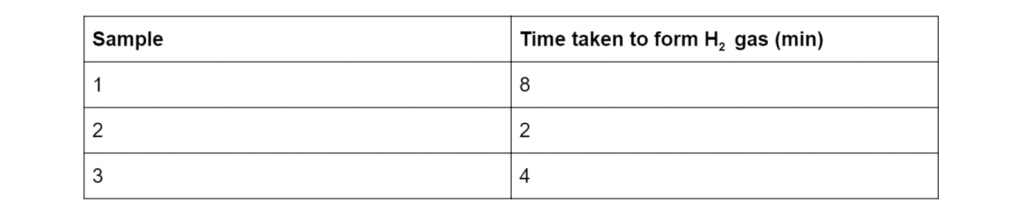
Question 12
Susan used sodium to identify primary, secondary and tertiary hydroxyl groups. Here are her results:
Identify which samples contain either primary, secondary and tertiary hydroxyl groups (3 marks).
(L2.1: Conduct qualitative investigations to test for the presence of organic molecules of the following functional groups: Carbon-carbon double bonds, Hydroxyl groups, Carboxylic acids)
Question 13
What is the importance of calcium hydroxide when testing for the presence of carboxylic acid using sodium carbonate? (3 marks)
(L2.1: Conduct qualitative investigations to test for the presence of organic molecules of the following functional groups: Carbon-carbon double bonds, Hydroxyl groups, Carboxylic acids)
Question 14
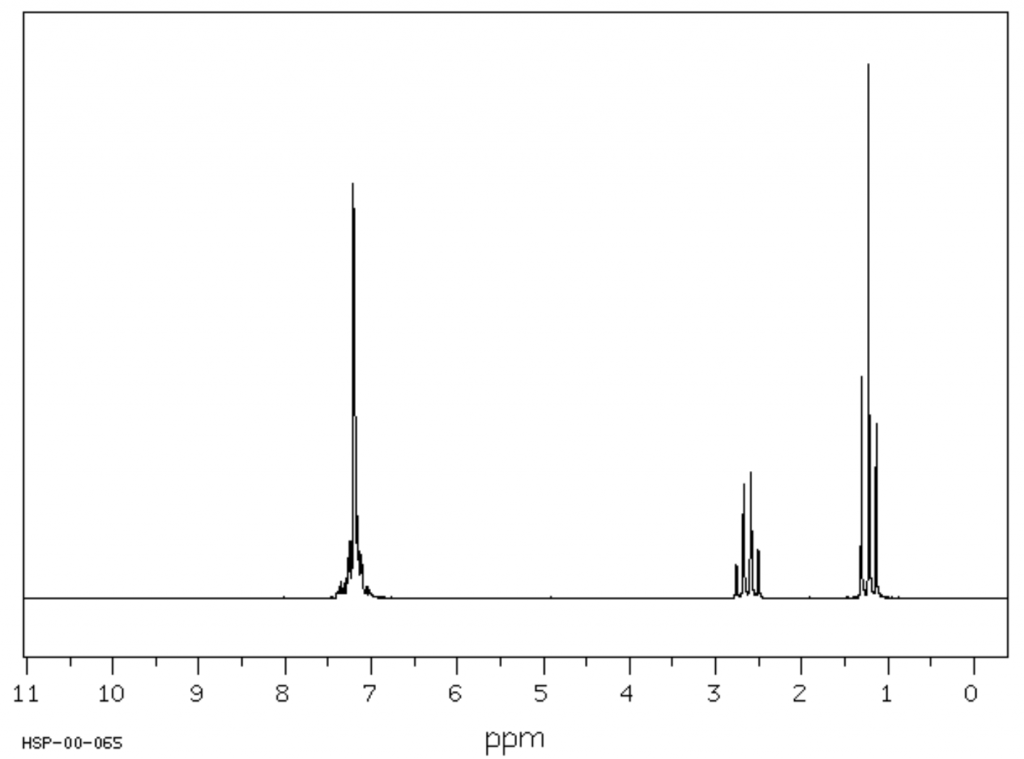
Identify the organic compound using the 1H NMR graph below. (3 marks)
Image sourced from eBioChemicals
(L2.2: Investigate the processes used to analyse the structure of simple organic compounds addressed in the course, including but not limited to: Proton and carbon-13 NMR, Mass spectroscopy, Infrared spectroscopy)
Question 15
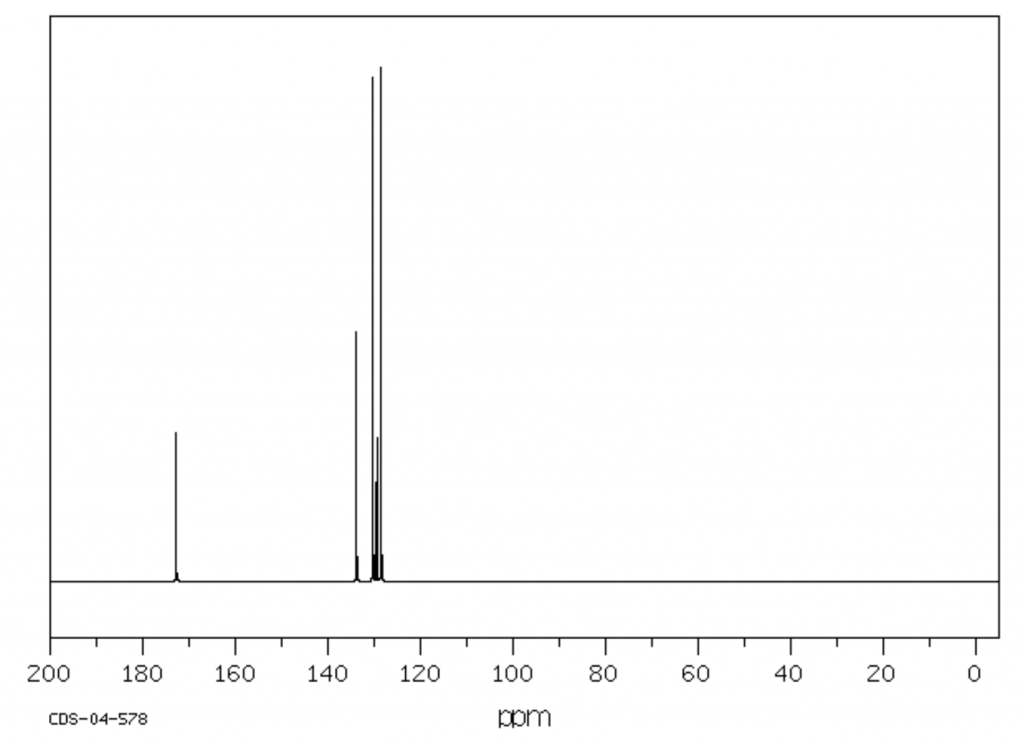
Identify the structural formula of the organic compound illustrated in the ¹³C graph below. (3 marks)
Image sourced from Chemical Book
(L2.2: Investigate the processes used to analyse the structure of simple organic compounds addressed in the course, including but not limited to: Proton and carbon-13 NMR, Mass spectroscopy, Infrared spectroscopy)
Question 16
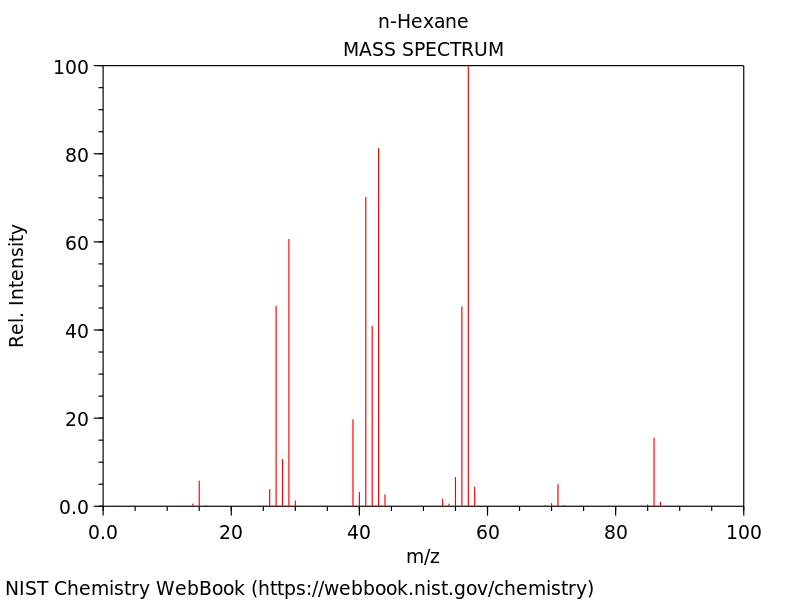
Identify the structural formula using the following mass spectroscopy graph. (3 marks)
(L2.2: Investigate the processes used to analyse the structure of simple organic compounds addressed in the course, including but not limited to: Proton and carbon-13 NMR, Mass spectroscopy, Infrared spectroscopy)
Question 17
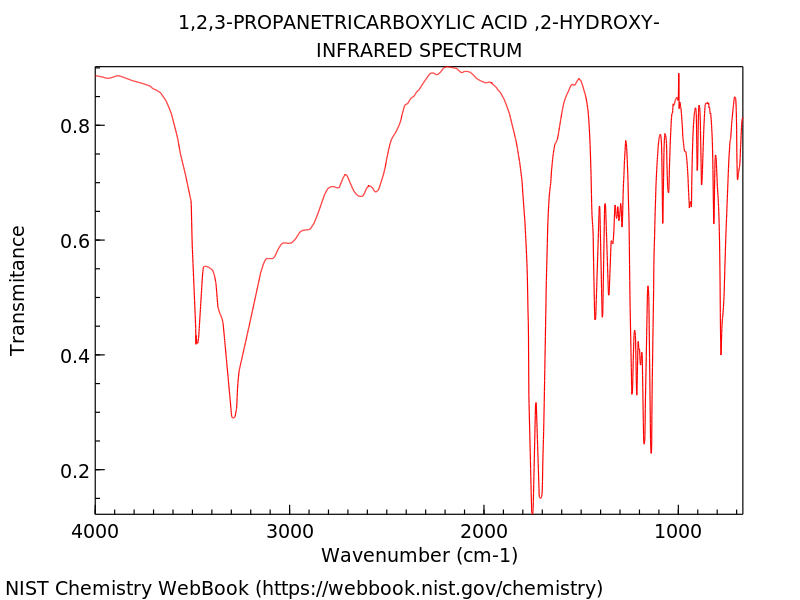
Identify the organic compound using the infrared spectrum graph below. (3 marks)
(L2.2: Investigate the processes used to analyse the structure of simple organic compounds addressed in the course, including but not limited to: Proton and carbon-13 NMR, Mass spectroscopy, Infrared spectroscopy)
Chemical Synthesis and Design
Question 18
Consider the Haber process, whereby atmospheric nitrogen and hydrogen are converted synthetically into ammonia as described in the equilibrium equation below:
Evaluate the importance of monitoring the availability of reagents and reaction conditions in this process with relation to yield and purity. (7 marks).
(L3.1: Evaluate the factors that need to be considered when designing a chemical synthesis process, including but not limited to: Availability of reagents, Reaction conditions, Yield and purity, Industrial uses, Environmental, social and economic issues)
Question 19
Describe examples of industrial uses of products that are made from a named chemical synthesis process. (4 marks)
(L3.1: Evaluate the factors that need to be considered when designing a chemical synthesis process, including but not limited to: Availability of reagents, Reaction conditions, Yield and purity, Industrial uses, Environmental, social and economic issues)
Question 20
Evaluate the importance of considering environmental, social and economic factors when designing a chemical synthesis process. Include a specific example of a chemical synthesis process and its relevant chemical equations. (7 marks)
(L3.1: Evaluate the factors that need to be considered when designing a chemical synthesis process, including but not limited to: Availability of reagents, Reaction conditions, Yield and purity, Industrial uses, Environmental, social and economic issues)
And that wraps up our 20 practice questions to HSC Chemistry Module 8: Apply Chemical Ideas – good luck!
Aiming for a Band 6 in HSC Chemistry? Check out our guide to scoring one here!
Looking for some extra help with Applying Chemical Ideas?
We have an incredible team of HSC Chemistry tutors and mentors who are new HSC syllabus experts!
We can help you master the HSC Chemistry syllabus and ace your upcoming HSC Chemistry assessments with personalised lessons conducted one-on-one in your home or at one of our state of the art campuses in Hornsby or the Hills!
We’ve supported over 8,000 students over the last 11 years, and on average our students score mark improvements of over 20%!
To find out more and get started with an inspirational HSC Chemistry tutor and mentor, get in touch today or give us a ring on 1300 267 888!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.