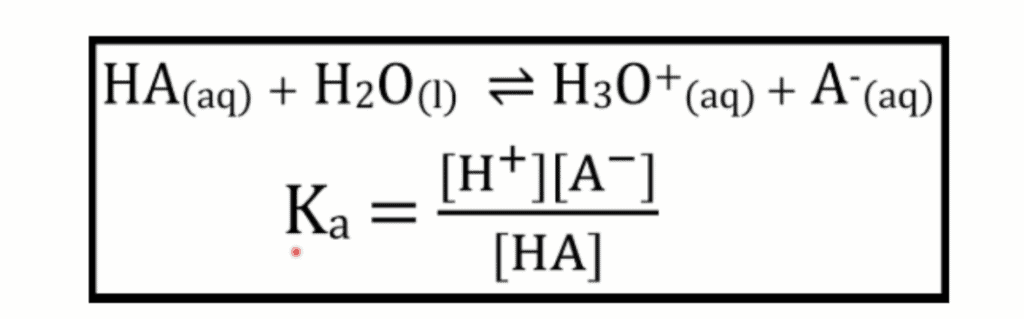Struggling with the content from HSC Chemistry Module 6: Acid/Base Reactions? Don’t sweat it!
In Year 12 Chemistry, acids and bases extend beyond flavour and litmus papers as we start looking at acids and bases at a much more molecular scale.
While there are many definitions of acids and bases (which you will learn later on in this module), acids are commonly known as proton donators while bases are proton acceptors.
These acids and bases are very important to our body, industry and environment, as much of life depends on pH, a measure of the concentration of protons produced by acids or consumed by bases.
In this article, we’ll be breaking down each of the inquiry questions in HSC Chemistry Module 6: Acid/Base reactions plus give you our top 3 tips on how to get a Band 6 in this module!
So, what are you waiting for? Let’s get to it!
Overview of Module 6: Acid/Base Reactions
How to get a Band 6 in HSC Chemistry Module 6
Overview of Module 6: Acid/Base Reactions
Before we dive into each of the inquiry questions, if you need to review concepts from HSC Chemistry, make sure you head over to HSC Together here which has FREE video resources explaining concepts within each syllabus dot point!
Inquiry Question 1: What is an acid and what is a base?
The first step to mastering anything is to learn the basics first! (No pun intended).
Here, we review what we know about acids and bases from junior high school. Acids are sour and bases are bitter. Easy right?
Remember, Year 12 extends junior high knowledge. While we merely scraped off balancing equations in junior high, it is now important to remember the products of each of the following reactions:
- Acid + Base → Salt + Water
- Acid + Carbonate → Salt + Carbon dioxide + water
- Acid + Metal → Salt + Hydrogen
Neutralisation, the addition of acid and base together is particularly important, and you would need to revise Module 4: Drivers of Reaction to understand the thermodynamics of neutralisation.
Hint: Neutralisation is exothermic. Can you explain why?
Additionally, there are two different definitions for both acids and bases; the Arrhernius theory and the Bronsted-Lowry theory.
| Arrhernius theory | Bronsed-Lowry theory |
|---|---|
| - Acids ionize in water to form protons (H+) - Bases ionize in water to form hydroxide ions (OH-) | - Acids are proton donators - Bases are proton acceptors |
It is important to know the definitions and their limitations accurately, as many of the trickier Band 6 questions would target this area of your understanding!
Inquiry Question 2: What is the role of water in solutions of acids and bases?
Acids and bases are good friends with water.
This is because water allow concentrations to be made. These concentrations will become pivotal in determining pH and pOH, a measure of acidity and basicity, respectively.
pH = -log10[H+]
pOH = – log10 [OH-]
Where [ ] is concentration in mol/L
pH + pOH = 14
Where pH =1 is most acidic and pH = 14 is most basic.
It is important to know what a low pH or high pH means. To avoid confusion, here is a summary of what a high pH means and what a low pH means and etc.
| pH | pOH | |
|---|---|---|
| High | Low concentration of H+ Low acidity High pH | Low concentration of OH- Low basicity Low pH |
| Low | High concentration of H+ High acidity Low pH | High concentration of OH- High basicity High pH |
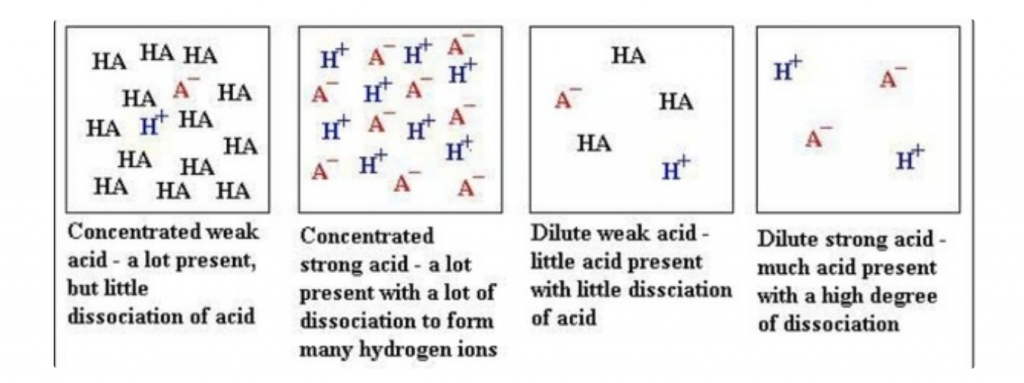
Yet, pH or pOH can be complicated depending on the strength of acids and bases
NB: Strength of acid/base is defined as its degree of dissociation.
A strong acid/base completely dissociates in water while a weak acid/base does not completely dissociate in water.
In the case of a weak acid/base, this decreases the concentration of H+ that increases the pH and decreases the concentration of pOH that decreases the pH.
Try not to get confused between strength and concentration of an acid/base. A concentrated or diluted acid/base has nothing to do with its strength.
Sourced from Quora
Inquiry Question 3: How are solutions of acids and bases analysed?
Now we know how to measure the concentration of strong acids/bases, how do we do the same for weak acids/bases?
Here is where titration comes in!
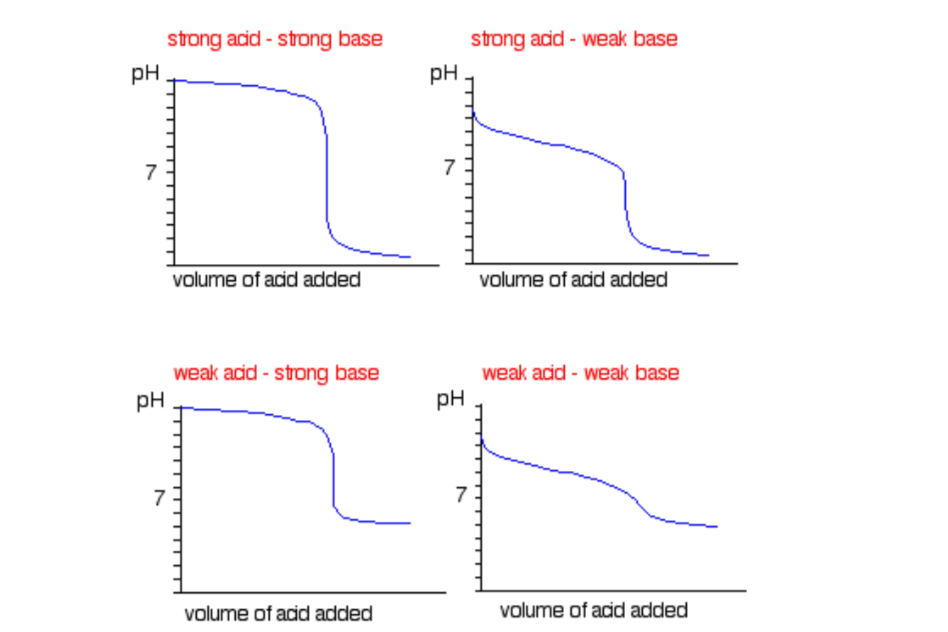
Titration is a technique that uses a solution of known concentration to measure the concentration of an unknown solution. This technique can be used for:
- Strong acids and strong base pairs
- Strong acids and weak base pairs
- Weak acids and strong base pairs
These pairs have their own unique titration curves and indicators used! Be sure to remember them! To help, here is a summary:
Sourced from ChemGuide
Note: Titrations of weak acids and weak bases are not required of Year 12 level so don’t sweat it!
| Titration pairs | Best Indicators | Indicator end point pH range |
|---|---|---|
| Strong acid and strong base | Phenolphthalein | 8.3-10 |
| Strong acid and weak base | Methyl Orange | 3.1-4.4 |
| Weak acid and strong base | Phenolphthalein | 8.3-10 |
Most importantly, the new syllabus have introduced a new formula to quantitatively measure the difference between strong and weak acids, the dissociation constant, Ka.
Note: Most weak acid/base reactions are in equilibrium because they do not dissociate completely. Meanwhile, strong acid/base reactions are not in equilibrium because their reaction goes to completion.
Ka is the measure of the dissociation extent of the acid. It is expressed as:
Ka looks very similar to Keq, and in fact, Ka ACTS like Keq too. It indicates whether the equilibrium reaction goes forward or backward.
The bigger the Ka, the more the acid dissociates, the stronger the acid.
Alternatively, the smaller the Ka, the less the acid dissociates, the weaker the acid.
How to get a Band 6 in HSC Chemistry Module 6: Acid/Base Reactions
Tip #1: Map out acids and bases
Whew! Despite having only three inquiry questions, Module 6 does carry some heavy concepts!
To organise everything you know, compile them into a mind map that centralises on acids and bases!
Design your mind map such that each branch addresses each inquiry question.
This way you can visually grasp how one concept relates to another, forming an umbrella summary that can be used for revision for future assessments!
For this module, be sure to include all necessary formulas as calculations play a pivotal role in acids and bases.
Tip #2: Master calculations
For examiners, Module 6: Acid/Base Reaction is a treasure box of calculation questions.
There are calculations within titration, pH, pOH, pKa and diluted or concentrated solutions.
To prepare yourself for the trickiest of calculations, the best way is to practise, practise, practise.
This way, you not only form a routine to approaching same styled questions, but also expose yourself to the more unique questions that require a bit of thinking outside the box!
Lucky for you, these calculations have not changed much from the old syllabus’ “Acidic Environment”. Therefore, past HSC papers are an amazing resource!
Not enough time to complete papers? No worries.
The workbook, Checkpoint Chemistry is an all-encompassing resource that includes the trickiest short and long response questions by the topic.
So what are you waiting for? Dig in!
Tip #3: Apply theory to practicals and real life situations
Acid and base reactions occur all around us!
That is why the new syllabus requires you to apply acid/base theories to industrial, environmental and even biological reactions.
Be sure to also apply the theory you have learnt in class to the practicals you’ve done, especially for titration!
For practise, ask yourself these two questions when revising the practical applications of acids and bases:
- What are the properties of acids or bases that contribute to its uses?
- How do the properties of acids and bases contribute to its uses?
By asking yourself these questions, you’re basically mastering your understanding of chemistry and how they are related to real life!
And that wraps up our guide to HSC Chemistry Module 6: Acid/Base Reactions! Good luck!
Need help in your other Chemistry modules? Check out our other guides below!
- Guide to HSC Chemistry Module 5: Equilibrium and Acid Reactions
- Guide to HSC Chemistry Module 7: Organic Chemistry
Looking for some extra help with HSC Chemistry?
We provide Specialists in Personalised HSC Chemistry Tutoring Sydney!
We offer tutoring and mentoring for Years K-12 in a variety of subjects, with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby!
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on Facebook!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.