Struggling to find practice questions for the new HSC Chemistry syllabus Module 6: Acid-Base Reactions?
You’ve come to the right place!
Despite being new, the HSC Chemistry Module 6: Acid-Base Reactions actually draws most of its learning dot points from the old syllabus.
You might find the same neutralisation reactions, titration concepts and acid/base equilibrium calculations that were spread across all modules of the old syllabus, now condensed into one thick module.
Haven’t finished your study notes for Module 5 yet? We’ve created a complete guide to Equilibrium and Acid Reactions for you, so don’t skip it!
Luckily for you, we’ve compiled 20 HSC Chemistry practice questions that cover each of the 20 learning dot points from Module 6: Acid-Base Reactions for you to practice with. Couple them with these HSC Chemistry Study Tips and you’ll be that much closer to a Band 6!
Enjoy!
Properties of Acids and Bases
Brønsted–Lowry
Quantitative Chemistry
Properties of Acids and Bases
Question 1
Justify why transporting concentrated sulfuric acid is more favourable than transporting diluted sulfuric acid. Use relevant chemical equations. (3 marks)
(L1.1:investigate the correct IUPAC nomenclature and properties of common inorganic acids and bases)
Question 2
Assess the validity and accuracy of the red cabbage indicator experiment. (4 marks)
(L1.2: conduct an investigation to demonstrate the preparation and use of indicators as illustrators of the characteristics and properties of acids and bases and their reversible reactions)
Question 3
Write balanced chemical equations to predict the products of:
- Phosphoric acid and sodium hydroxide
- Sulfuric acid and sodium bicarbonate
- Hydrochloric acids and magnesium metal
(L1.3: predict the products of acid reactions and write balanced equations to represent: – acids and bases – acids and carbonates – acids and metals)
Question 4
Justify the importance of neutralization reactions in everyday life and industrial processes. Give at least 2 examples for each. (5 marks)
(L1.4: investigate applications of neutralisation reactions in everyday life and industrial processes)
Need some help consolidating your Chemistry content? Our experienced Chemistry tutors near you provide tailored tutoring support in the comfort of your own home or online.
Question 5
Draw the experimental set-up you have performed in class to measure the enthalpy of neutralization. Describe how the value you have obtained is different to the theoretical value of neutralization of -56kJ/mol and explain why. (6 marks)
(L1.5: conduct a practical investigation to measure the enthalpy of neutralisation)
Question 6
Justify the continued use of the Arrhenius definition of acids and bases, despite the development of the more sophisticated Brønsted–Lowry definition. (4 marks)
(L1.6: explore the changes in definitions and models of an acid and a base over time to explain the limitations of each model, including but not limited to: – Arrhenius’ theory – Brønsted-Lowry theory)
Using Brønsted-Lowry Theory
Question 7
Complete the table below (3 marks)
Indicator Colour in Acid Colour in Base Methyl Orange Bromothymol blue Phenophathalein (L2.1: conduct a practical investigation to measure the pH of a range of acids and bases)
Question 8
A solution was made by mixing 75.00 mL of 0.120 mol L-¹ hydrochloric acid with 25.00 mL of 0.200 mol L-¹ sodium hydroxide. (4 marks)
What is the pH of the solution?
(L2.2: calculate pH, pOH, hydrogen ion concentration ([H+ ]) and hydroxide ion concentration ([OH– ]) for a range of solutions)
Question 9
Using your understanding of weak and strong acids, explain how the pH of 0.1M of hydrochloric acid would differ from 0.1M of sulfuric acid. (3 marks)
(L2.3: conduct an investigation to demonstrate the use of pH to indicate the differences between the strength of acids and bases)
Question 10
Using ionic equations, explain the amphiprotic nature of sodium hydrogen carbonate or potassium dihydrogen phosphate according to the Bronsted-Lowry definition. (4 marks)
(L2.4: write ionic equations to represent the dissociation of acids and bases in water, conjugate acid/base pairs in solution and amphiprotic nature of some salts, for example: – sodium hydrogen carbonate – potassium dihydrogen phosphate)
Question 11
Referencing the model you have used in class to communicate differences between strong and weak acids, explain why strong acids have a lower pH compared to the weak acids of the same concentration. (4 marks)
(L2.5: construct models and/or animations to communicate the differences between strong, weak, concentrated and dilute acids and bases)
Question 12
500mL of 0.1M hydrochloric acid has been diluted to a volume of 125mL. The resultant diluted solution was then mixed with 200mL of 0.1M NaOH. Find the pH of the solution. (3 marks)
(L2.6: calculate the pH of the resultant solution when solutions of acids and/or bases are diluted or mixed)
Quantitative Chemistry
Question 13
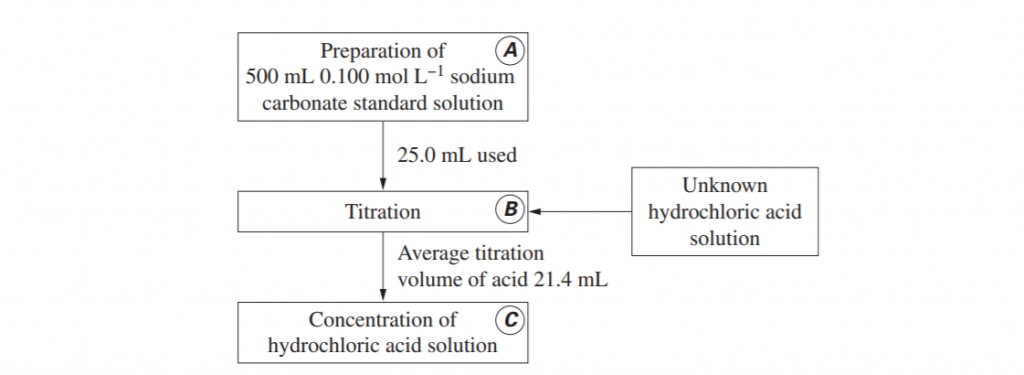
The flowchart shown outlines the sequence of steps used to determine the concentration of an unknown hydrochloric acid solution.
Describe steps A, B and C including correct techniques, equipment and appropriate calculations. Determine the concentration of the hydrochloric acid. (8 marks)
(L3.1: conduct practical investigations to analyse the concentration of an unknown acid or base by titration)
Question 14
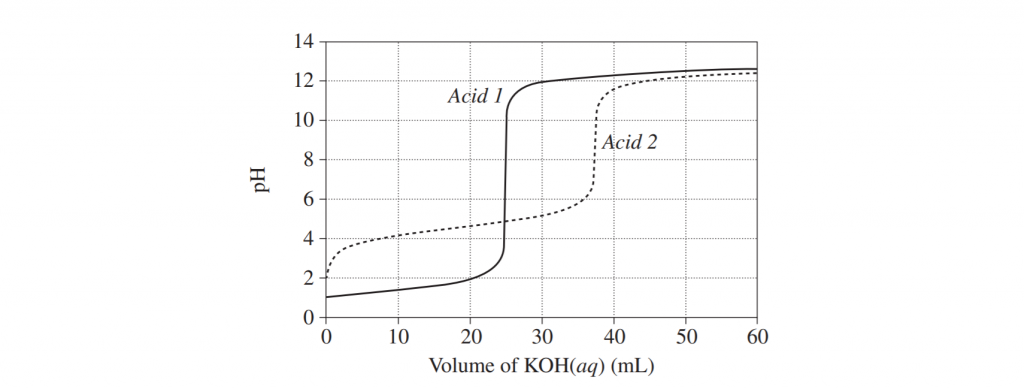
The graph shows changes in pH for the titrations of equal volumes of solutions of two monoprotic acids, Acid 1 and Acid 2.
a) Explain the difference between Acid 1 and Acid 2 in terms of their relative strengths and concentrations. (3 marks)
b) Name the salt produced by the reaction of an acid of the same type as Acid 2 with KOH(aq) has been added to Acid 1. (1 mark)
c) Why would phenolphthalein be a suitable indicator for both titrations? (1 mark)
d) Why would phenolphthalein be a suitable indicator for both titrations? (1 mark)
(L3.2: investigate titration curves and conductivity graphs to analyse data to indicate characteristic reaction profiles, for example:
– strong acid/strong base
– strong acid/weak base
– weak acid/strong base)
Question 15
In class, you have modelled the neutralization of strong and weak acids and bases using different medias. Explain how the use of scientific models can enhance our understanding of reactions on a molecular scale? (4 marks)
(L3.3: model neutralisation of strong and weak acids and bases using a variety of media)
Question 16
A monoprotic acid solution with a concentration of 0.01M has a pH of 4.8 at 25 degrees. Calculate the value of Ka and state whether this acid is likely to be a strong acid or weak acid? (3 marks)
(L3.4: calculate and apply the dissociation constant (Ka) and pKa (pKa = -log10 (Ka)) to determine the difference between strong and weak acids)
Question 17
Outline 3 ways acids or bases are used within the industry and Aboriginal and Torres Strait Islander people. (3 marks)
(L3.5: explore acid/base analysis techniques that are applied: – in industries – by Aboriginal and Torres Strait Islander Peoples – using digital probes and instruments)
Question 18
Sam wants to investigate whether the following items in his household are acidic or basic: Juice, detergent and water. Outline a method he can carry out to test its acidity/ basicity. (3 marks)
(L3.6: conduct a chemical analysis of a common household substance for its acidity or basicity, for example: – soft drink – wine – juice – medicine)
Question 19
Using your understanding of equilibrium, explain how a named buffer works when an acid or a base is added. (4 marks)
(L3.7: conduct a practical investigation to prepare a buffer and demonstrate its properties)
Question 20
Explain the role of the conjugate acid/base pair, H₂PO₄- /HPO₄²-, in maintaining the pH of living cells. Include chemical equations in your answer.
(L3.8: describe the importance of buffers in natural systems)
That rounds off our 20 HSC Chemistry practice questions for Module 6: Acid-Base Reactions! Good luck!
Looking for some extra help with HSC Chemistry?
We pride ourselves on our inspirational HSC Chemistry coaches and mentors!
We offer tutoring and mentoring for Years K-12 in a variety of subjects, with personalised lessons conducted one-on-one in your home or at our state of the art campus in Hornsby!
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on Facebook!
Kate Lynn Law graduated in 2017 with an all rounders HSC award and an ATAR of 97.65. Passionate about mentoring, she enjoys working with high school students to improve their academic, work and life skills in preparation for the HSC and what comes next. An avid blogger, Kate had administrated a creative writing page for over 2000 people since 2013, writing to an international audience since her early teenage years.