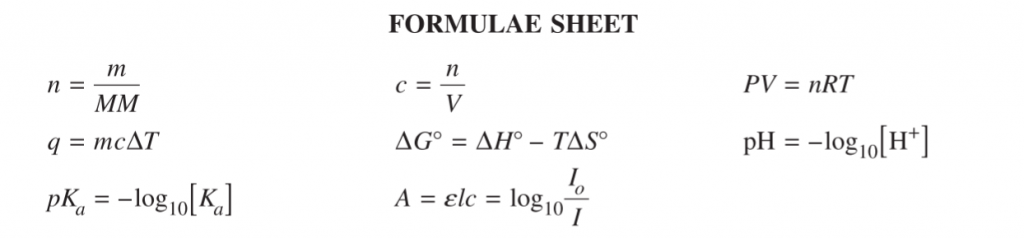Antsy about your HSC Chemistry Exam? We’re to help you navigate the HSC Chemistry exam paper!
We’ve got step-by-step explanations for each type of question you can anticipate in your exam.
Let’s dive in!
Changes to the HSC Chemistry Syllabus
Structure of the HSC Chemistry Exam
Section 1: Multiple Choice Questions
Section 2: Modules
How to Ace a 5-9 Mark Question
Changes to the HSC Chemistry Syllabus
Change #1: The Word Count is Down
The 8 modules (Year 11 and 12) of old-syllabus chemistry were about 8900 words worth of raw content dot-points.
The new syllabus is about 4800 words — that’s a 46% reduction in syllabus wordiness!
What that meant with the old syllabus is that you could study-up on a stack of ideas really solidly and have some of them, tragically, never even show up in the HSC Chemistry exam as winnable marks.
Because the new syllabus focuses on the mastering the core concepts in Chemistry, it’s way easier to anticipate which ideas the HSC Chemistry exam will test you on them.
In other words, there should be way less questions in the HSC Chemistry exam that you don’t know about!
Looking to score a Band 6 this year? Start using these study tips for HSC Chemistry to see your results improve!
Change #2: More of a Calculations Focus
Where the syllabus declares something to be calculated, it’s telling you practice, practice and practice those calculations!
Here’s the whole lot of them across the whole two years:
Here’s what the top B your formulae sheet will look like from the HSC Chemistry exam:
Chemistry Formulae Sheet Sourced from NESA05
Change #3: Everyone Learns the Same Content
For the first time in two decades, you will do the EXACT same chemistry course as everyone else at every other school in the entire state.
The HSC Chemistry syllabus is much more similar to what all the other Chemistry students are learning in other states in Australia — and, in fact, all the other countries in the world!
Structure of the HSC Chemistry Exam
Chemistry Sample Paper Sourced From NESA
Note: Section I is entirely multiple choice questions while Section II consists of short answer and extended responses.
A few things regarding the format jump out straight away:
- You’ve got 1.8 minutes per mark on average. This is the same as the past HSC Chemistry exam papers.
- The multiple-choice section is 20% of the paper (in marks and time). This is the same as before.
- The 80% extended response section is the rest of the paper and it’s the same questions or everybody (there is no “option” topic part to this).
So, let’s have a look at these sections!
Section 1: Multiple Choice Questions
The easiest parts of the HSC Chemistry exam live in this section – but that also means they’re the easiest to mess up because you’re off-guard.
They’re simple but still worth 20 marks, so make sure you don’t rush.
There are 20 multiple-choice questions, one mark each, and the recommended time allocation is 35 minutes. For your 20 multiple-choice questions, you have 1.5 minutes for each question.
That said, if you can finish the multiple-choice section in 30 minutes, this will free up some planning time in the modules.
What Would a Band 6 Student Do?
Well-practised students will smash some of the questions immediately (particularly when 3 of the 4 options can be excluded).
This allows you to extra time for the back of the paper where more answer-planning and deep-thinking come in to play.
If you’re running something like the -10% model, where you aim to finish the exam with 10% of time spare, this can be a great place to win time.
Note: Try not spend two minutes on any of these multiple-choice questions until you’ve made your first pass through the rest of the paper.
If you do the paper in order and start with the MCQs, remember:
- Your chemistry brain (and your brain chemistry) is still warming up
- You’re at your highest anxiety level at the start – you’re still worrying about the rest of the paper.
If you find yourself spending 2 minutes on any multiple-choice question, come back to them when you’ve got the bulk of the rest of the paper in the bag already.
Some of the troublesome MCQs will look different when you view them with a brain that’s been in the zone for 2 hours!
Got 5 minutes? Check out our 3 quick steps to acing HSC Chemistry here!
Types of Multiple Choice Questions in the HSC Chemistry Exam
Roughly speaking, there are three types of questions you’ll get in MCQs:
- Fact Checking
- Concept Checking
- Calculation
How to Answer a Fact Checking Question
Fact-checking types are the easiest marks in the HSC Chemistry exam.
All they require is that you recall a fact, and pick the right answer out of the four.
Consider Question 3 from NESA’s sample HSC Chemistry exam:
2019 Chemistry Sample paper Sourced From NESA
You’ll win this one by being well-versed in some facts.
Question 3 (above, left) looks quite busy, but it’s actually as straightforward as it gets – you either know how to draw out the structures of the listed chemical species from their names, or you don’t (which you clearly do because you studied diligently); you either know the absorbance wave numbers of the functional groups, or you don’t.
That can make it a hard question in a sense: There’s not a lot of room to logic yourself in the direction of the answer. You can’t really apply a tool to the problem to deduce the answer.
These questions test whether you’ve read the textbook enough?”. They’re in every HSC exam and measure how much you’ve studied throughout the year.
For the well-practised student, these questions are “gift-marks”.
How to Answer a Concept Checking Question
Multiple-choice questions also include concept-checking questions.
These are a little bit harder, because they require you not just to recall, but to think.
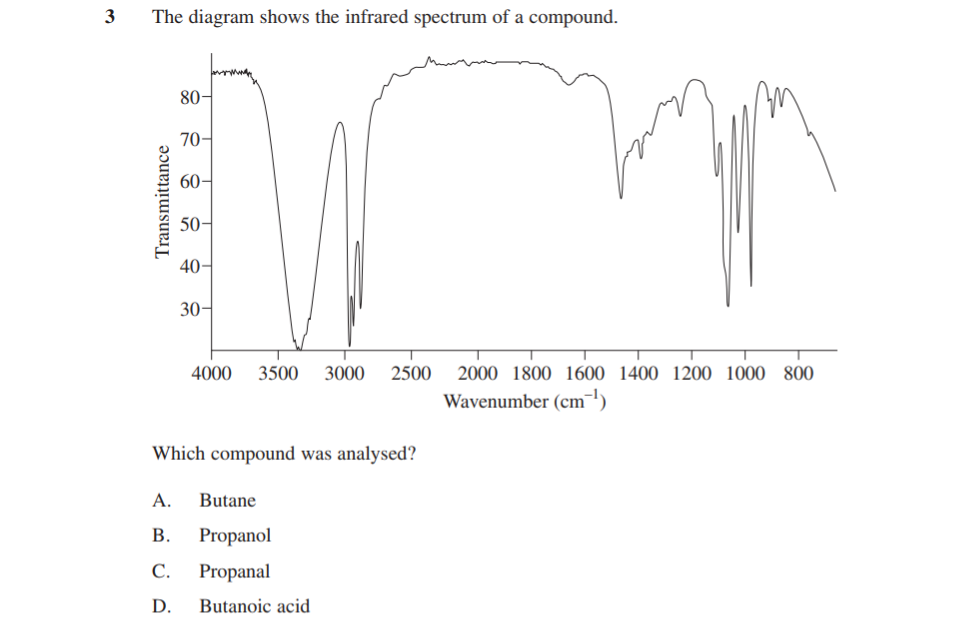
Take a look at Question 2:
2019 Chemistry Sample Paper Sourced From NESA
You’ll have to bring deep understanding of concepts to this one as this one cannot be won by glancing at it and knowing.
First things first, to answer this one you need to appreciate (on a conceptual level) the difference between a system in static equilibrium and one in dynamic equilibrium
When zoomed-out, the question is testing if you understand how kinetic particle theory gives rise to the reality observed at a macro-level (what humans see).
To answer this question, you’ll need to use some logic. Since Question 2 is a concept-checking question, it has to be worked out by applying understanding of ideas.
To find the answer, we need the following information:
Static equilibrium: The stable state of the system emerges because none of its constituents change – for this reason they stay in fixed relative amounts.
e.g. a system that completely depletes one of its reactants and none of the product molecules fall apart.
Dynamic equilibrium: The stable state of the system emerges because the system rate-matches its interconversion of products and reactants, keeping their relative amounts the same overall.
e.g. A system that rate-matches the forwards and backwards reactions, so therefore must have stores of both available at equilibrium.
Based on this logic:
- D cannot be the answer as both panels depict static equilibrium
- C cannot be the answer as the right panel depicts dynamic equilibrium (the reaction will still be turning reactant in to product, since both reactants are still present in quantity)
- B cannot be the answer as each panel depicts the opposite type of equilibrium
- A is the answer (confirm this to yourself as a way of checking the logic applied to the other answer options)
See how, in this type of question, there’s a lot more than just picking out one essential fact?
It takes some active thinking on your part.
Check out more Acid and Equilibrium practice questions here!
Calculation Questions
The concept-checking question is similar to the calculation question. But these are generally a bit easier.
This is because there are not many different calculations you can be asked to do. So, you can study and get ready for all of them!
Let’s have a look at another example:
2015 HSC Chemistry Exam Sourced From NESA
Note: This question is of a Band-6 Level. Expect Q19 & Q20 to be at about this level in the real exam.
This is a calculation question, but it is quite involved. The key to calculation questions is to make every mistake possible handling them prior to the exam.
By the time you arrive at this HSC question, you should be highly-experienced at solving these type of problems. All the mistakes you could have made have already been done!
But, you can actually solve this one without a calculator (what a time-saver!)
Remember, since pKa = – logKa, a high pKa flags an acid as dissociating to a lesser extent. If you zoomed into the solution, you’d still find intact acid molecules all over the place when the pKa is high.
But why?
Because weak acids leave behind pretty strong conjugate bases that are really good at picking those H+ ions back up and re-building the acid as it tries to fall apart (even ripping protons off passer-by water molecules in the solvent).
Then, a solution of a salt of a strong conjugate base will remove hydrogen from water (as it does in a solution of its conjugate acid), forming OH– in solution.
This will be the solution with the highest pH: The one with the strongest base (associated with an acid with the highest pKa)
Answer: C
Some questions (not all) are calculation-based but can be won with raw logic. Other questions are just purely computational.
So, use them to help you work backwards.
- If there’s an answer that’s definitely wrong, cross it out.
- If there’s one that’s the odd one out from the other four, it’ll either be the right answer or very, very, wrong!
- If you can narrow down your choices, you may even be able to find the right answer by taking out three wrong ones, or at least narrow your chances to take an educated guess between two.
Tip: You just need to identify the most correct answer out of the four provided.
Tips for Multiple Choice Questions
Generally speaking, the answer you pick first is probably correct.
Statistically, when you change your answer, it’s more common not to gain a mark or even lose one, than it is to gain one.
These questions are designed to be easy, so trust your gut!
Also, expect a difficulty gradient through the MCQs with Question 20 often at a Band 6 level. The Band 6 level Question will take more than 1.8 minutes (and worth coming back to when you’re sure you have the time – and also when your brains warmed up!)
Most of all, set your own goalposts for timing – make sure you’re allocating resources where you need them!
Note: The options often involve 1 correct answer and three “trap” answers, which NESA created by guessing at common ways to trip over the question.
Section 2: Modules
Offering 80 marks, the modules is the bulk of the HSC Chemistry exam paper – give yourself more than two hours for them.
While the recommended time is 2 hours and 25 minutes, this section runs on well-planned, well-thought-out and well-phrased extended answers.
So, try to get 2 and a half hours by smashing out your MCQs!
Note: If you want some time to go over your questions after you’ve finished the paper, you might want to do this in 2 hour and 10 minutes.
Just like we subdivided the MCQs, this section is best divided:
- 1-2 Mark Questions
- 3-4 Mark Questions
- 5+ Mark Questions
1-2 Mark Questions
While the previous HSC papers used to be littered with 1-2 mark questions that asked you to “identify” something (essentially, regurgitate from the textbook), these “identify” questions won’t come up in the 2022 HSC Chemistry Exam.
Instead, you’ll require a deeper level of thinking as the syllabus focuses on task words, including “explain”, “analyse” and “deduce”.
3-4 Mark Questions (Mid-Range Questions)
3-4 markers take a bit more work.
They tend to come in two varieties:
- Skill questions, involving things like graphing or experiment design
- Word questions, where you need to write a little diatribe.
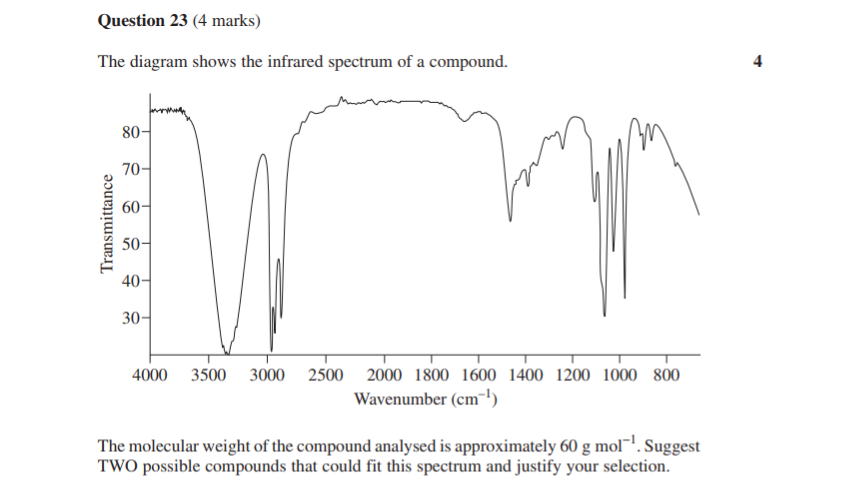
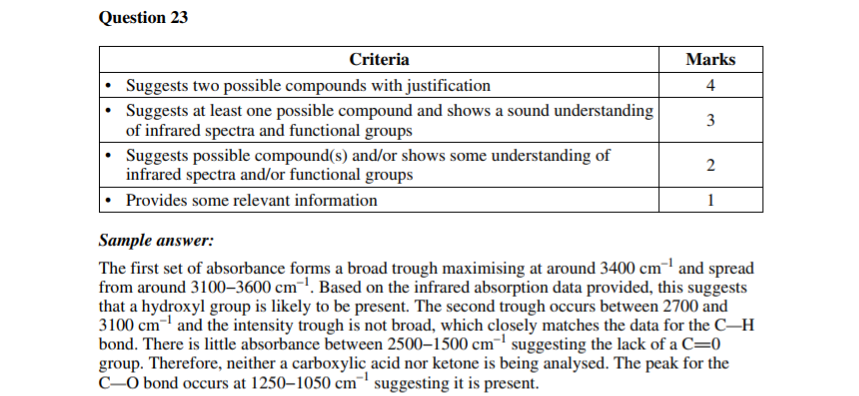
Let’s take a look at skills questions first. This one here involves an IR spectrum.
If you’re super switched-on, you’ll notice this is kind of a concept-checking version of the Question 3 we looked at from the MCQs, where all you had to do was ID the compound.
This time you have to “justify” two possible answers as the question asks; and this also requires you to know some facts (absorbance wavelengths).
2019 Chemistry Sample Paper Sourced From NESA
While the criteria doesn’t spell it out, you get one mark for each of 4 requirements.
For example, in this case it’s likely a mark for the right answer (two structures) and then 3 marks for correctly justifying the structure from each of three absorbances (shown or conspicuously absent from the spectrum).
2019 Chemistry Sample Paper Criteria Sourced From NESA
Have a month until your HSC Chemistry exam? Check out our 7 Day HSC Chemistry Study Plan here!
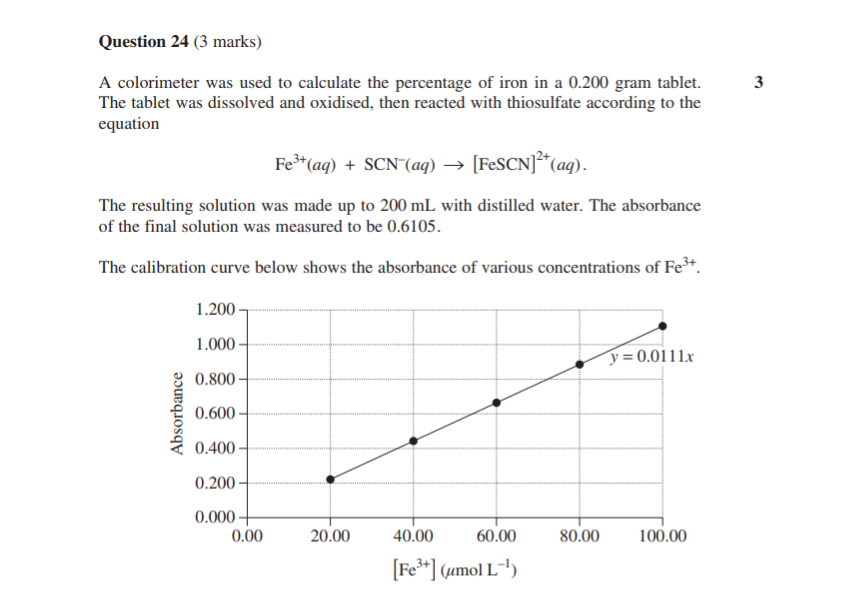
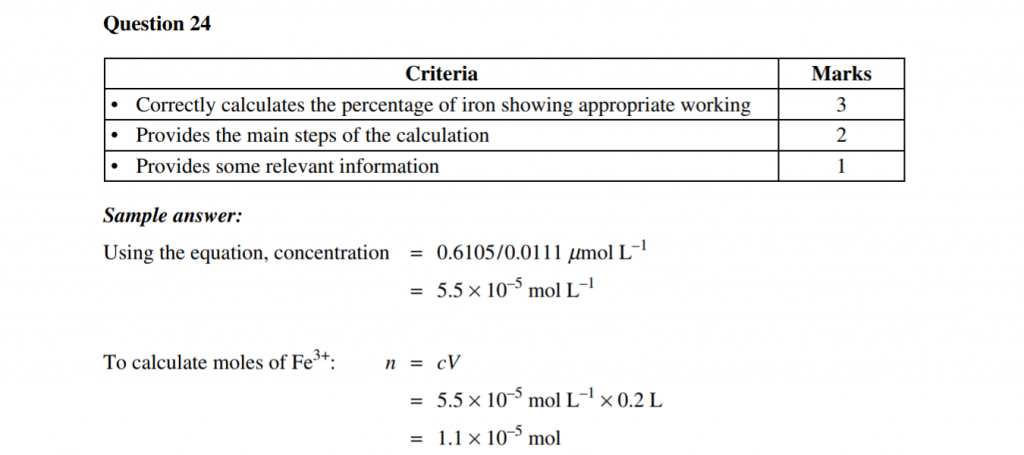
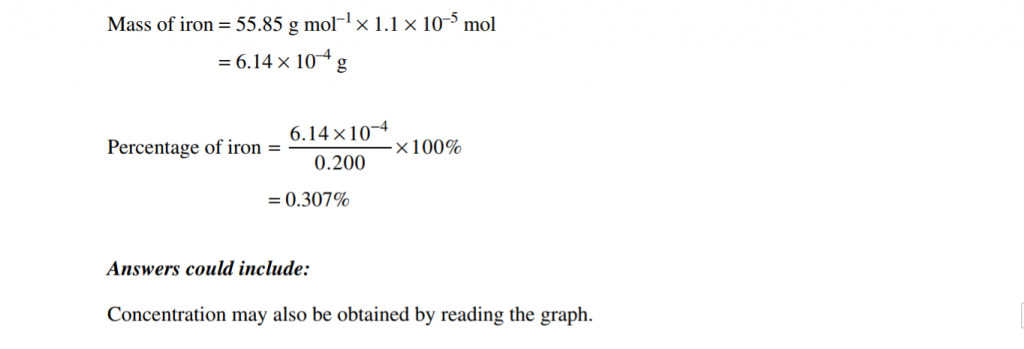
Let’s look at a question aimed at your graphing skills:
2019 Chemistry Sample Paper Sourced From NESA
2019 Chemistry Sample Paper Criteria Sourced From NESA
Note: You could also have determined the Fe3+ concentration by ruling some lines and carefully reading it from the graph.
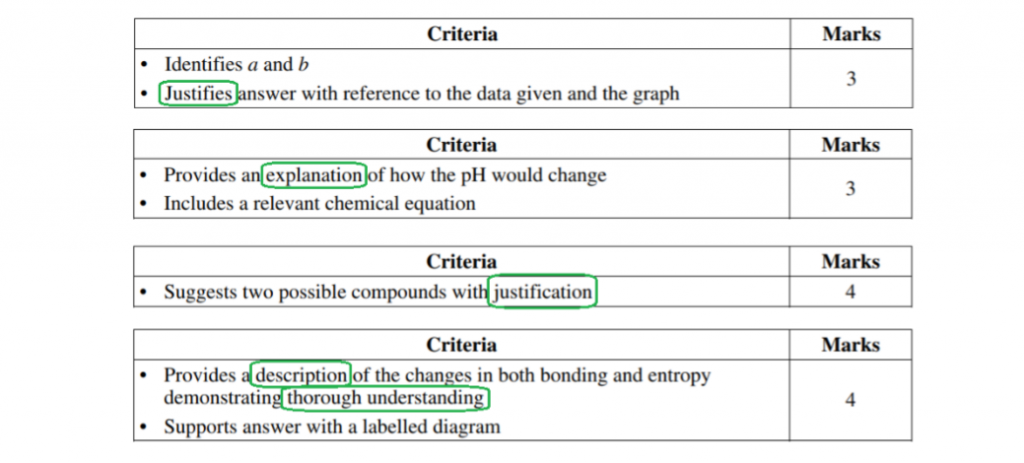
3-4 markers tend to use verbs like “describe” and “explain” and “justify”.
These are verbs that involve not only recall of facts but ask you to draw connections between several facts, often cause-and-effect.
Often these require you to draw links between the fundamental chemistry (i.e. what’s going on at the atomic level) and the effect that we actually observe.
Here’s a stack of marking criteria from NESA’s sample HSC Chemistry exam for the new syllabus, with a clear verb pattern:
5-9 Markers: The Heavyweights
5+ markers are what separate the Band 6s from the 5s.
Basically, they involve the high-order verbs such as:
- Discuss: Provide pros and cons
- Assess: Make a judgement as to the value of
- Evaluate: Make a judgement based on criteria
Make sure to spend some time planning your answer before jumping in.
You only have limited space to convey lots of information! As an example:
2019 Chemistry Sample Paper and Criteria Sourced From NESA
Notice the quality gradient in the marking criteria above for high-end scorers.
See how the verb slips downwards:
- Explain: Relate cause and effect; make the relationships between things evident; provide why and/or how
- Describe: Provide characteristics and features)
- Outline: Sketch in general terms; indicate the main features of
You can grab the rest of the terms and definitions here!
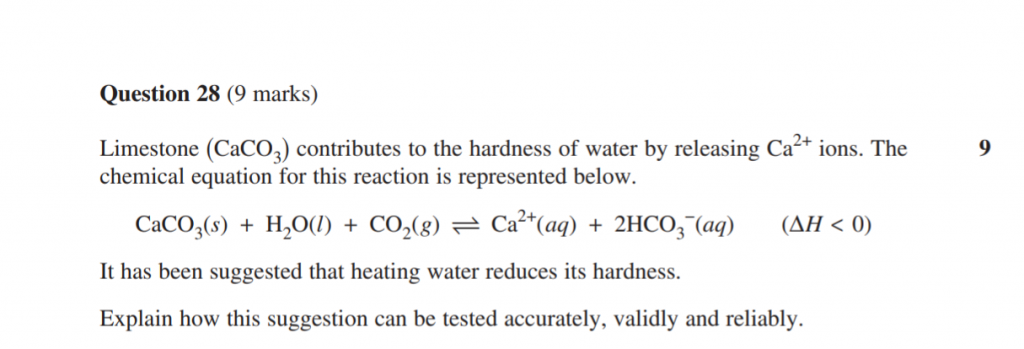
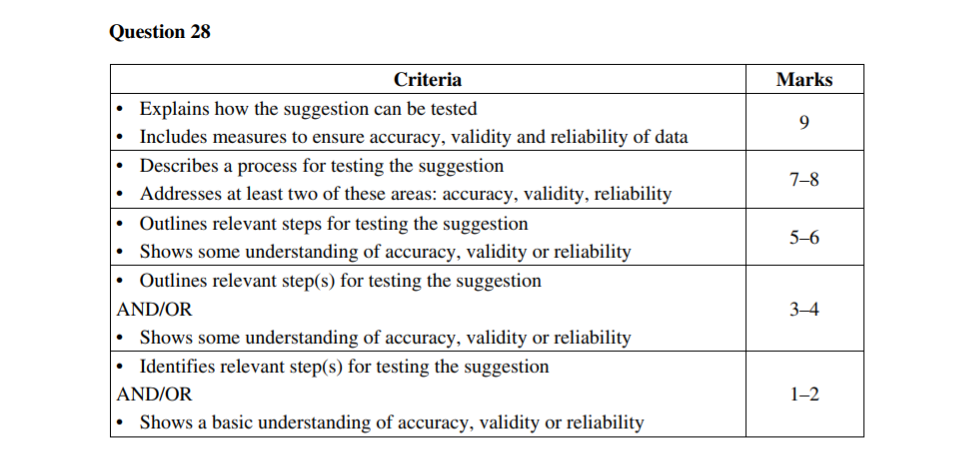
For the full 9 marks, you have to not only know the meanings of accuracy, validity and reliability and how they’re different. But, you also had to include experimental methods that would enhance each of these (i.e. all three).
Slip down to 8 marks, and you only addressed two terms from accuracy, validity, reliability.
Tip: As a general note for this section, try not to go over the allotted number of lines for your answer — particularly if the rest of the page is blank.
The exam writer allocated that number of lines for a reason, and that reason was to try and keep your answer concise.
If you go wildly over the line-space, you haven’t treated other questions with adequate time somewhere in the rest of the paper.
How to Ace a 5-9 Mark Question
Your answer here is like a mini-essay.
You have to address the question directly and quite methodically.
If we treat it like an essay, you would have a structure with an introduction outlining the main points and the main points paragraphed out. Finally, you have a summary statement that brings it all home and sums up each element the question wanted you to address.
Step 1: Outline Main Aspects of the Question
To plan these, start by outlining the main aspects of the question.
That way when you’re about to write your conclusion statement, you can return to the question and it’s immediately clear if you’ve actually addressed every point the question wanted you to (It’s easy to forget what the question was when you’re writing away in the line space over on the next page!)
Step 2: Structure and Write Your Answer
Structure your answer using the main aspects you underlined. Some questions are begging for you to use sub-headings. Don’t be afraid to dot-point some things in to a list if that would express the point more clearly than a block of text.
As a general rule, you can never win all the marks in a heavy-marker without several chemical equations (always include some real chemistry in your word answer!); and if something is more easily explained graphically (as a diagram of apparatus or a flowchart), incorporate that in your answer.
If you’re agonising over phrasing and struggling to articulate a chemical concept mid-way through a wordy mini-essay, switch in to table/diagram/dot-point mode.
So that’s the HSC Chemistry Exam in a Nutshell!
We know the HSC Chemistry exam will look like a lot.
But, knowledge is power.
With this guide on hand and more HSC Chemistry resources below, practice and go into this exam with bulletproof confidence:
- HSC Chemistry Module 7: Organic Chemistry Practice Questions
- HSC Chemistry Past Papers Master List
- HSC Chemistry: The Ultimate Guide to a Band 6
- HSC Chemistry Data Breakdown: Drop Rates, Band 6 Distribution & more
- How Should I Study for HSC Chemistry?
Looking for some extra help with your HSC Chemistry Exam?
We pride ourselves on our inspirational coaches and mentors!
We offer tutoring and mentoring for Years K-12 in a variety of subjects, with personalised lessons conducted one-on-one in your home or at one of our state of the art campuses in Hornsby or the Hills!
To find out more and get started with an inspirational tutor and mentor get in touch today!
Give us a ring on 1300 267 888, email us at [email protected] or check us out on TikTok!
Matt Saunders is a huge nerd who first got into writing through fanfiction. He’d known science was the path for him since a young age, and after discovering a particular love of bad chemistry jokes (and chemistry too), he’s gone onto to study Forensic Chemistry at UTS. His HSC in 2014 was defined in equal parts by schoolwork and stagecraft, which left him, weirdly enough, with a love of Maths strong enough to inspire him to tutor any level, along with 7-10 Science and HSC Chemistry.
Adrian Wendeborn is a qualified science and maths teacher with a physics/chemistry double-major degree from USYD and a GDipEd from UQ. Adrian has taught in QLD and NSW and has worked with Art of Smart Education as a campus teacher, tutor, resource developer and Head of Faculty.